Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Rodica Turcu.
Bio-magnetic nanoparticles (BMNPs) was introduced, describing a unique combination of physio-chemical properties of magnetic nanoparticles with their entirely biocompatible nature, which makes them particularly effective in various biomedical applications.
- magnetic nanomaterials
- cancer
- drug delivery systems
- targeted therapy
1. Introduction
Cancer represents a major public health problem worldwide, being the first leading cause of death in people below the age of 70 in North America, Canada, Australia, China, and numerous European countries [1]. With the increase in population and life expectancy, it is predicted that the incidence of cancer will rise in the following years, reaching 28.4 million cases annually worldwide by 2040 [2]. In this context, finding effective therapies for cancer is crucial for reducing the medical and economic burden of this disease.
Conventional cancer therapies include surgery, radiotherapy, and/or chemotherapy. Among these, chemotherapy is widely used but presents various side effects that can limit patient compliance and adherence [3]. Moreover, cancer cells can develop chemotherapy resistance through many mechanisms, rendering it ineffective [4]. Recently, non-coding RNAs such as micro RNAs (miRNA) and small interfering RNAs (siRNA) have been studied for their potential as novel cancer treatments due to their capacity to regulate gene transcription [5]. However, their applicability is limited too by their low stability, high costs, and immunological adverse reactions [6].
In recent years, nanotechnology has emerged as a tool for cancer treatment, promising improved therapeutic outcomes for both chemotherapy and RNA-based therapy, by delivering therapeutic agents in close proximity to the tumor using nano drug delivery systems (DDSs). Nano DDSs generally range in size between 10 and 100 nm, which allows sufficient circulation time and accumulation in the tumor tissue due to the enhanced permeability and retention (EPR) effect [7,8][7][8]. Numerous types of DDSs have been employed, such as polymers [9[9][10],10], lipid nanoparticles (NPs) [11], and metallic NPs [12] (silver, gold, or magnetic NPs). Among these, magnetic NPs represent a promising alternative, due to their properties such as high stability, high saturation magnetization/large magnetic moment of particles, good response to moderate magnetic fields, inherent ability to cross biological barriers, protection of the drug from rapid degradation in biological systems, provision of a large surface area for conjugating targeting ligands [7,8,13,14,15,16,17,18[7][8][13][14][15][16][17][18][19],19], low production costs [20], and superparamagnetism, which allows their guidance in the organism using an external magnetic field [7,21][7][21]. Scientific interest in NPs in general and in magnetic nanoparticles (MNPs) in particular has grown exponentially in the last decade, due to recent high-interest research on their properties and the fact that in a relatively short time, these materials have become particularly important tools in high-interest biomedical areas such as biomaterial science, biochemistry, diagnostics, magnetic drug and gene delivery, hyperthermia, magnetic resonance imaging (MRI), and theragnostics [22,23,24,25,26,27,28,29,30,31][22][23][24][25][26][27][28][29][30][31]. The increased interest in the field of MNPs is demonstrated by the number of scientific articles provided by a simple “magnetic nanoparticles” keyword search in the Scopus database. Before 1995, fewer than 100 articles were published, but after 1996, when the first successful clinical trials took place, there was an exponential increase with 3000 articles in 2010, 6500 in 2015, and up to 8700 in 2020 [32]. As a general structure, NPs are considered inorganic or organic particles of submicron size with enhanced properties relative to a similar bulk form. The specific physical and chemical properties given by nanostructuring, such as optical, electrical, and magnetic properties or increased reactivity, made these special types of NPs attractive to nanotechnology. Following the development of biotechnological applications, the term “bio-magnetic nanoparticles” (BMNPs) was introduced, describing a unique combination of physio-chemical properties of magnetic nanoparticles with their entirely biocompatible nature, which makes them particularly effective in various biomedical applications [33]. Starting from the particular requirements of each biomedical application, BMNPs are considered to have a huge potential in drug delivery applications, because their surface can be specifically functionalized with various molecular layers [7,14,24,34][7][14][24][34]. Apart from drug delivery, magnetic NPs can be used for hyperthermia applications, MRI contrast imaging, and diagnosis procedures. Recently, magneto-mechanical actuation of MNPs has also been used as an anti-cancer strategy. In this technique, the application of a magnetic field does not lead to heating (such as in hyperthermia), but to vibrations of the MNPs in the proximity of cells, leading to mechanical alterations and cell death [35,36][35][36]. Moreover, complex strategies combining more of these approaches can also be employed. The combination of hyperthermia and drug delivery in the same carrier can enhance anti-cancer efficiency destroying cancer cells through multiple mechanisms.
2. Classification of BMNPs
In order to properly choose the most appropriate MNPs for specific biomedical applications, a very clear classification of them according to their nature, composition, and size is required, taking into account the synthesis methods used to prepare them and the specific functionalization of the surface. Over time, several types of MNPs have been developed and researched. Generally, these MNPs can be grouped into three main categories as (i) magnetic pure metals (Fe, Co, Ni), (ii) magnetic metal oxides (Fe2O3, Fe3O4) or ferrites (MeFe2O4, Me = Fe, Co, Zn), and (iii) multicomponent magnetic nanoparticles as core/shell MNPs or magnetic nanoclusters (Figure 1). Each of these mentioned categories has both advantages and disadvantages, but their properties can be adapted to fit a particular type of application. In the following, each category will be discussed and exemplified.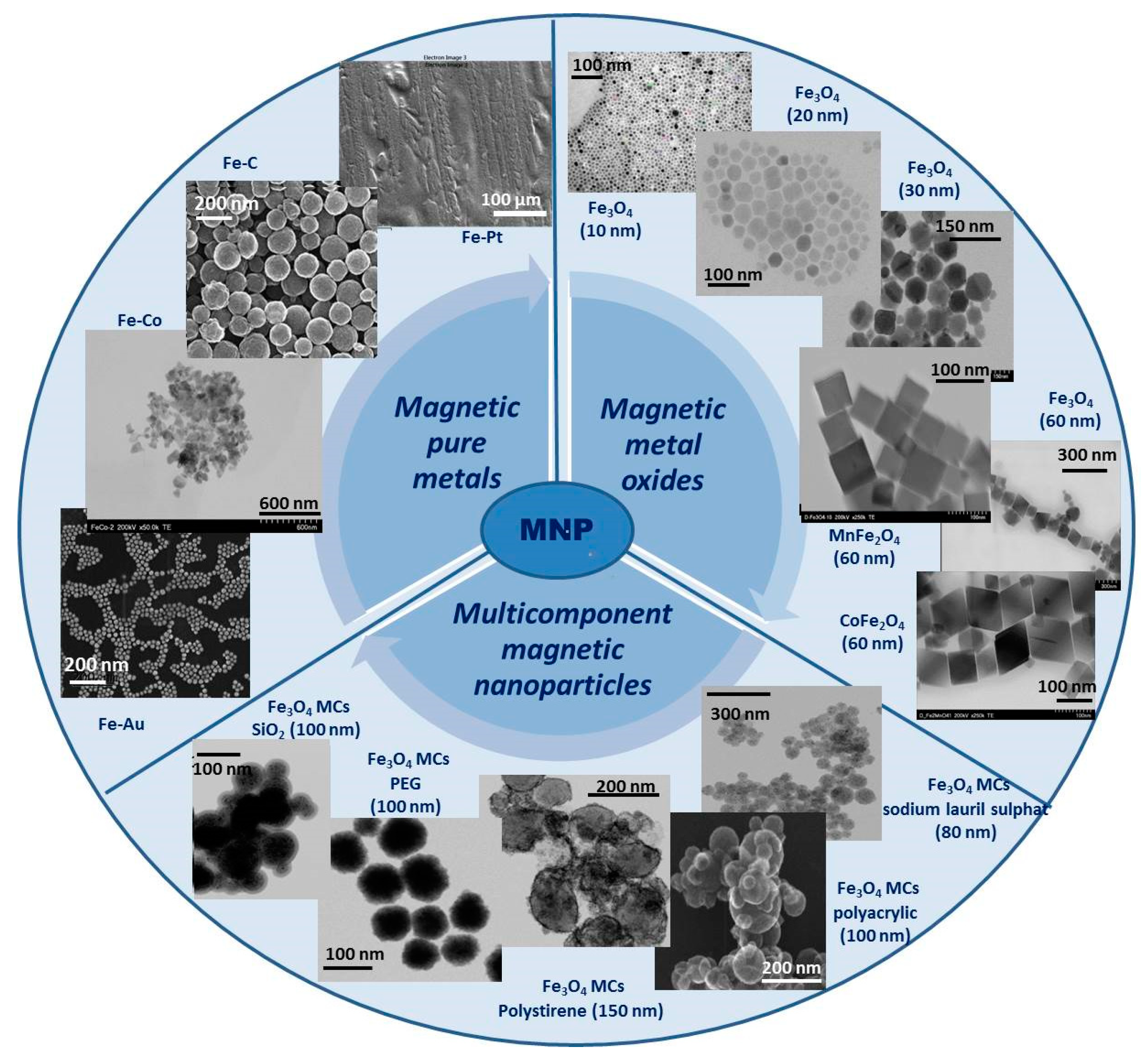
Figure 1. General classification and exemplification of magnetic nanoparticles (all images are original and belong to the researchers).
2.1. Magnetic Pure Metals
Pure metal type materials exhibit some unique properties, with some of them directly dependent on the distribution of electrons in the external orbitals, and magnetic behavior is one of these properties. Transition metals, such as Fe, Ni, Co, and Mn, are the most commonly used in this class because they show good magnetic performance in several biomedical fields [39][37]. Iron (Fe) nanoparticles are one of the most common ferromagnetic materials used for biomedical applications. The main advantage of using this material is related to its excellent magnetic properties, which can be exploited in a wide range of biomedical applications. In terms of synthesis methods, Fe nanoparticles can be obtained by relatively simple methods such as the reduction of iron salts in aqueous solutions in the presence of reducing agents such as sodium borohydride [40][38], or by thermal decomposition of Fe(CO)5 on a polymer matrix [41][39]. Even if the synthesis methods are facile and accessible, one of the major weaknesses of these NPs is that synthesis requires rigorous control of the surface-covering shell since direct contact between the Fe surface and air leads to their combustion. Therefore, the necessity to find homogeneous and uniform coatings for this kind of nanoparticle is critical. There are studies in which tailoring the reaction parameters and changing the Fe precursor to Fe[N(SiMe3)2]2 can result in major improvements in the reaction yield, better control of the size distribution, and a reduction in by-product formation [42][40]. In addition to Fe NPs, cobalt (Co) is another magnetic material commonly used in biomedical applications. Although its toxicity is higher than that of Fe NPs, beneficial effects of its use have been observed in specific MRI imaging applications and local hyperthermia of malignant tumors [43,44][41][42].2.2. Magnetic Metal Oxides
From the general class of metal oxides, iron oxide distinguishes itself as a material with excellent magnetic properties and low toxicity compared to pure metallic forms, which, due to its unique physio-chemical properties, has potential applications in nanotechnology [39][37]. Over time, metal oxides have gained popularity in biomedical applications due to their physicochemical and mechanical stability, low toxicity, and biocompatibility but also for their efficiency in some special areas of biomedical science such as magnetic bio-separation of some species of interest, magnetic hyperthermia, drug carriers at target sites, and bio sensing [45,46][43][44]. There are three types of iron oxides: Iron (III) oxide, with two subspecies: Hematite (α-Fe2O3) and maghemite (γ-Fe2O3); a rare type of iron (II) oxide (FeO) called wüstite; and iron (II, III) oxide named magnetite (Fe3O4). This different stoichiometry due to the flexibility of the Fe oxidation state (Fe2+/Fe3+) is supported by the formation of different single-crystal phases with different chemical and physical properties. Of all iron oxide structures, Fe3O4 has the most closely packed cubic inverted spinel structure and semi metallic properties, demonstrating great potential in biomedical fields [47][45]. One of the biggest advantages of these NPs is the ease of their synthesis at relatively high reaction yields by simple and conventional methods. In the beginning, conventional preparation methods such as the co-precipitation or solvothermal method [48,49,50,51,52,53][46][47][48][49][50][51] did not offer rigorous control over the size and size distribution of MNPs, so aggregation and poly dispersibility appeared, which hindered their use in biomedical applications. Over time, however, new synthesis methods were explored to obtain size-controlled NPs with a uniform size distribution. For example, by using organic iron precursors, Fe3O4 NPs with a narrow size distribution were obtained. By adjusting the molar ratio between metal precursors and different surfactant molecules, different particles can be obtained ranging in shape from cubic to polyhedral [54][52]. It can be concluded that the physicochemical properties of NPs, in particular the magnetic properties, can be adjusted according to the intended application with minimal changes in the composition and synthesis parameters. Although iron oxides have the advantage of easy preparation, they present certain limitations related to chemical stability under biological environmental conditions, often resulting in aggregation phenomena that decrease their potential use in biomedical applications. Coating the surface of iron oxide NPs with biocompatible molecular layers increases chemical and mechanical stability, reduces surface oxidation, and decreases toxicity in biological entities. Thus, iron oxide NPs, generically called superparamagnetic iron oxide nanoparticles (SPIONs), have been developed. These particles represent small synthetic iron oxide particles with a core ranging between 10 nm and 30 nm in diameter, coated with certain biocompatible molecules, which provide chemical handles for the conjugation of therapeutic agents and improve their blood distribution profile [55][53]. These particles exhibit superparamagnetic properties, meaning that under an external magnetic field, they magnetize to saturation magnetization, and when the magnetic field is removed, they exhibit no residual magnetic interaction. This property is dependent on the size of the NPs and generally occurs when their size is only 10–20 nm. At such a small size, these NPs do not exhibit multiple magnetic domains, as found in larger NPs, acting as a “single super spin” that exhibits high magnetic susceptibility. Thus, upon application of a magnetic field, these NPs provide a stronger and faster magnetic response compared to bulk magnets with negligible residual magnetization and coercivity (the field required to bring the magnetism to zero) [56][54]. This unique property of SPIONs represents a great advantage for their use in specific biomedical applications such as controlled drug delivery where these NPs function as drug delivery vehicles because they can drag specific drug molecules to the target site, under the influence of an external magnetic field. Furthermore, once the applied magnetic field is removed, the magnetic particles do not retain any residual magnetism and aggregation phenomena are avoided, thus evading absorption by phagocytes and increasing half-life in circulation. In addition, due to their negligible tendency to agglomerate, SPIONs pose no danger of thrombosis or blockage of blood capillaries [55][53]. A sub-division of metal oxides, emerging from the necessity of adjusting the properties of this type of material for specific biomedical applications, is spinel ferrites (MeFe2O4), a very important magnetic material due to their combined electrical and magnetic properties, which make them useful in many technological applications [57][55]. The main component of these materials is iron oxide doped with a variety of bivalent transition metals such as zinc (Zn), manganese (Mn), cobalt (Co), nickel (Ni), iron (Fe), platinum (Pt), and palladium (Pd). It has been demonstrated that due to the doping of the Fe3O4 structure with bivalent cations, ferrites show improved electrical and magnetic properties for hyperthermia applications, for example, but also present the disadvantage of higher toxicity compared to magnetite [58][56]. The use of some stabilizing molecules, which also confer biocompatibility on the ferrite NPs surfaces, is essential for biomedical practical applications. In the synthesis of Co ferrite NPs, a CoFe2(CO)9 precursor and two stabilizing agents, hexadecylamine and oleic acid, were used to obtain small, relatively polydisperse MNPs. When a third stabilizing agent, lauric acid, was introduced, bigger monodispersed crystalline structures with a narrower size distribution were obtained. A decrease in the toxicity of the system was also demonstrated, with the developed system showing promising applications against tumor cells [59][57]. Another particularly efficient approach to using ferrite NPs in biomedical applications is their coating with biocompatible polymer layers, such as polyhydroxy and polyamine-type polymers and polyethylene glycol (PEG). It has been proven that the use of these systems in controlled drug release applications is particularly effective, with researchers also performing biocompatibility tests, which demonstrated that these materials can be successfully applied without adverse effects regarding toxicity [60][58]. Other ferrite-type magnetic structures have been reported in the literature, which, in addition to excellent magnetic properties, also show lower toxicity, such as Zn-, Mn-, and Ni-based ferrites [61][59]. There are also ferrite-type systems, with two transition metal components, Co-Zn and Mn-Zn type, in which it was highlighted that the concentration of Zn ions has an effect on the size of the final particles, i.e., increasing the concentration of Zn cations decreases the size of the particles [62][60].2.3. Multicomponent Magnetic Nanoparticles
Multicomponent MNPs have been developed as a normal evolution of the technology because these systems can present multiple functionalities at the same time, by simply combining two or more components and thus offering new features that are not available in single-component materials or structures. In addition, in these multicomponent systems, improved specific properties can be obtained that may overcome the natural limitations of single-component materials. There are several types of multicomponent magnetic structures, but the most often studied and with real possibilities of application in biomedicine are the two main categories: (i) Core/shell type multicomponent MNPs and (ii) magnetic clusters (MCs).- (i)
-
Core/shell-type multicomponent magnetic nanoparticles
- (ii)
-
Magnetic clusters
- (a)
-
Single step-magnetic cluster procedures
- (b)
-
Multi-step magnetic cluster procedures
References
- World Health Organization. Global Health Estimates (2020). Available online: who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 3 March 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Cancer Chemotherapy-StatPearls-NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564367/ (accessed on 15 February 2023).
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233.
- Cuciniello, R.; Filosa, S.; Crispi, S. Novel approaches in cancer treatment: Preclinical and clinical development of small non-coding RNA therapeutics. J. Exp. Clin. Cancer Res. 2021, 40, 383.
- Zare, M.; Pemmada, R.; Madhavan, M.; Shailaja, A.; Ramakrishna, S.; Kandiyil, S.P.; Donahue, J.M.; Thomas, V. Encapsulation of miRNA and siRNA into Nanomaterials for Cancer Therapeutics. Pharmaceutics 2022, 14, 1620.
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-the-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 236.
- Chavan, N.; Dharmaraj, D.; Sarap, S.; Surve, C. Magnetic nanoparticles–A new era in nanotechnology. J. Drug Deliv. Sci. Technol. 2022, 77, 103899.
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4.
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403.
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109.
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615.
- Sankaranarayanan, S.A.; Thomas, A.; Revi, N.; Ramakrishna, B.; Rengan, A.K. Iron oxide nanoparticles for theranostic applications-Recent advances. J. Drug Deliv. Sci. Technol. 2022, 70, 103196.
- Mai, B.T.; Conteh, J.S.; Gavilán, H.; Di Girolamo, A.; Pellegrino, T. Clickable Polymer Ligand-Functionalized Iron Oxide Nanocubes: A Promising Nanoplatform for “Local Hot Spots” Magnetically Triggered Drug Release. ACS Appl. Mater. Interfaces 2022, 14, 48476–48488.
- Mekseriwattana, W.; Guardia, P.; Herrero, B.T.; de la Fuente, J.M.; Kuhakarn, C.; Roig, A.; Katewongsa, K.P. Riboflavin-citrate conjugate multicore SPIONs with enhanced magnetic responses and cellular uptake in breast cancer cells. Nanoscale Adv. 2022, 4, 1988–1998.
- Mishra, S.K.; Herman, P.; Crair, M.; Constable, R.T.; Walsh, J.J.; Akif, A.; Verhagen, J.V.; Hyder, F. Fluorescently-tagged magnetic protein nanoparticles for high-resolution optical and ultra-high field magnetic resonance dual-modal cerebral angiography. Nanoscale 2022, 14, 17770–17788.
- Aires, A.; Fernández-Afonso, Y.; Guedes, G.; Guisasola, E.; Gutiérrez, L.; Cortajarena, A.L. Engineered Protein-Driven Synthesis of Tunable Iron Oxide Nanoparticles as T1 and T2 Magnetic Resonance Imaging Contrast Agents. Chem. Mater. 2022, 34, 10832–10841.
- Portilla, Y.; Fernández-Afonso, Y.; Pérez-Yagüe, S.; Mulens-Arias, V.; Morales, M.P.; Gutiérrez, L.; Barber, D.F. Different coatings on magnetic nanoparticles dictate their degradation kinetics in vivo for 15 months after intravenous administration in mice. J. Nanobiotechnol. 2022, 20, 543.
- Yan, B.; Wang, S.; Liu, C.; Wen, N.; Li, H.; Zhang, Y.; Wang, H.; Xi, Z.; Lv, Y.; Fan, H.; et al. Engineering magnetic nano-manipulators for boosting cancer immunotherapy. J. Nanobiotechnol. 2022, 20, 547.
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J. Control. Release 2021, 335, 437–448.
- kianfar, E. Magnetic Nanoparticles in Targeted Drug Delivery: A Review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735.
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Sel. 2021, 2, 1146–1186.
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic nanoparticles for biomedical purposes: Modern trends and prospects. Magnetochemistry 2020, 6, 30.
- Socoliuc, V.; Peddis, D.; Petrenko, V.I.; Avdeev, M.V.; Susan-Resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vékás, L. Magnetic nanoparticle systems for nanomedicine—A materials science perspective. Magnetochemistry 2020, 6, 2.
- Hepel, M. Magnetic nanoparticles for nanomedicine. Magnetochemistry 2020, 6, 3.
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003.
- Mittal, A.; Roy, I.; Gandhi, S. Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry 2022, 8, 107.
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243.
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 188.
- Chouhan, R.S.; Horvat, M.; Ahmed, J.; Alhokbany, N.; Alshehri, S.M.; Gandhi, S. Magnetic nanoparticles—A multifunctional potential agent for diagnosis and therapy. Cancers 2021, 13, 2213.
- Bruschi, M.L.; de Alcântara Sica de Toledo, L. Pharmaceutical Applications of Iron-Oxide. Magnetichemistry 2019, 5, 50.
- Petrov, K.; Chubarov, A. Magnetite Nanoparticles for Biomedical Applications. Encyclopedia 2022, 2, 1811–1828.
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845.
- Stanicki, D.; Vangijzegem, T.; Ternad, I.; Laurent, S. An update on the applications and characteristics of magnetic iron oxide nanoparticles for drug delivery. Expert Opin. Drug Deliv. 2022, 19, 321–335.
- Nikitin, A.A.; Ivanova, A.V.; Semkina, A.S.; Lazareva, P.A.; Abakumov, M.A. Magneto-Mechanical Approach in Biomedicine: Benefits, Challenges, and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 11134.
- Naud, C.; Thébault, C.; Carrière, M.; Hou, Y.; Morel, R.; Berger, F.; Diény, B.; Joisten, H. Cancer treatment by magneto-mechanical effect of particles, a review. Nanoscale Adv. 2020, 2, 3632–3655.
- Nosheen, S.; Irfan, M.; Abidi, S.H.; Syed, Q.; Habib, F.; Asghar, A.; Waseem, B.; Soomro, B.; Butt, H. Mubashar Akram A review: Development of magnetic nano vectors for biomedical applications. GSC Adv. Res. Rev. 2021, 8, 85–110.
- Yoon, T.J.; Shao, H.; Weissleder, R.; Lee, H. Oxidation kinetics and magnetic properties of elemental iron nanoparticles. Part. Part. Syst. Charact. 2013, 30, 667–671.
- Farrell, D.; Majetich, S.A.; Wilcoxon, J.P. Preparation and Characterization of Monodisperse Fe Nanoparticles. J. Phys. Chem. B 2003, 107, 11022–11030.
- Dumestre, F.; Chaudret, B.; Amiens, C.; Renaud, P.; Fejes, P. Superlattices of iron nanocubes synthesized from Fe2. Science 2004, 303, 821–823.
- Petit, C.; Taleb, A.; Pileni, M.P. Cobalt nanosized particles organized in a 2D superlattice: Synthesis, characterization, and magnetic properties. J. Phys. Chem. B 1999, 103, 1805–1810.
- Murray, C.B.; Sun, S.; Doyle, H.; Betley, T. Monodisperse 3d Transition-Metal (Co, Ni, Fe) Nanoparticles and Their Assembly intoNanoparticle Superlattices. MRS Bull. 2001, 26, 985–991.
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415.
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265.
- Quinto, C.A.; Mohindra, P.; Tong, S.; Bao, G. Multifunctional superparamagnetic iron oxide nanoparticles for combined chemotherapy and hyperthermia cancer treatment. Nanoscale 2015, 7, 12728–12736.
- Zhu, Y.; Zhao, W.; Chen, H.; Shi, J. A simple one-pot self-assembly route to nanoporous and monodispersed Fe3O4 particles with oriented attachment structure and magnetic property. J. Phys. Chem. C 2007, 111, 5281–5285.
- Xuan, S.; Wang, F.; Wang, Y.X.J.; Yu, J.C.; Leung, K.C.F. Facile synthesis of size-controllable monodispersed ferrite nanospheres. J. Mater. Chem. 2010, 20, 5086–5094.
- Xuan, S.; Wang, Y.X.J.; Yu, J.C.; Leung, K.C.F. Tuning the grain size and particle size of superparamagnetic Fe 3O4 microparticles. Chem. Mater. 2009, 21, 5079–5087.
- Liu, J.; Sun, Z.; Deng, Y.; Zou, Y.; Li, C.; Guo, X.; Xiong, L.; Gao, Y.; Li, F.; Zhao, D. Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew. Chemie-Int. Ed. 2009, 48, 5875–5879.
- Fang, X.L.; Chen, C.; Jin, M.S.; Kuang, Q.; Xie, Z.X.; Xie, S.Y.; Huang, R.B.; Zheng, L.S. Single-crystal-like hematite colloidal nanocrystal clusters: Synthesis and applications in gas sensors, photocatalysis and water treatment. J. Mater. Chem. 2009, 19, 6154–6160.
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chemie-Int. Ed. 2005, 44, 2782–2785.
- Zeng, H.; Rice, P.M.; Wang, S.X.; Sun, S. Shape-Controlled Synthesis and Shape-Induced Texture of MnFe2O4 Nanoparticles. J. Am. Chem. Soc. 2004, 126, 11458–11459.
- Wahajuddin; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomedicine 2012, 7, 3445–3471.
- Dave, S.R.; Gao, X. Monodisperse magnetic nanoparticles for biodetection, imaging, and drug delivery: A versatile and evolving technology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 583–609.
- Hossain, A.; Sarker, M.S.I.; Khan, M.K.R.; Khan, F.A.; Kamruzzaman, M.; Rahman, M.M. Structural, magnetic, and electrical properties of sol–gel derived cobalt ferrite nanoparticles. Appl. Phys. A 2018, 124, 608.
- Veiseh, O.; Sun, C.; Fang, C.; Bhattarai, N.; Gunn, J.; Kievit, F.; Du, K.; Pullar, B.; Lee, D.; Ellenbogen, R.G.; et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009, 69, 6200–6207.
- Lee, J.H.; Huh, Y.M.; Jun, Y.W.; Seo, J.W.; Jang, J.T.; Song, H.T.; Kim, S.; Cho, E.J.; Yoon, H.G.; Suh, J.S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99.
- Osorio-Cantillo, C.; Santiago-Miranda, A.N.; Perales-Perez, O.; Xin, Y. Size- and phase-controlled synthesis of cobalt nanoparticles for potential biomedical applications. J. Appl. Phys. 2012, 111, 07B324.
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012, 324, 903–915.
- Ben Ali, M.; El Maalam, K.; El Moussaoui, H.; Mounkachi, O.; Hamedoun, M.; Masrour, R.; Hlil, E.K.; Benyoussef, A. Effect of zinc concentration on the structural and magnetic properties of mixed Co-Zn ferrites nanoparticles synthesized by sol/gel method. J. Magn. Magn. Mater. 2016, 398, 20–25.
- Kortan, A.R.; Hull, R.; Opila, R.L.; Bawendi, M.G.; Steigerwald, M.L.; Carroll, P.J.; Brus, L.E. Nucleation and Growth of CdSe on ZnS Quantum Crystallite Seeds, and Vice Versa, in Inverse Micelle Media. J. Am. Chem. Soc. 1990, 112, 1327–1332.
- Lee, H.; Yoon, T.J.; Weissleder, R. Ultrasensitive Detection of Bacteria Using Core Shell Nanoparticles and an NMR-Filter. Angew. Chem. Int. Ed. Engl. 2009, 48, 5657–5660.
- Lee, I.S.; Lee, N.; Park, J.; Kim, B.H.; Yi, Y.-W.; Kim, T.; Kim, T.K.; Lee, I.H.; Paik, S.R.; Hyeon, T. Ni/NiO Core/Shell Nanoparticles for Selective Binding and Magnetic Separation of Histidine-Tagged Proteins. J. Am. Chem. Soc. 2006, 128, 10658–10659.
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjal, G.A.; Alivisatos, A.P. Formation of Hollow Nanocrystals Through the Nanoscale Kirkendall Effect. Science 2004, 304, 711–714.
- Ha, D.-H.; Moreau, L.M.; Bealing, C.R.; Zhang, H.; Hennig, R.G.; Robinson, R.D. The structural evolution and diffusion during the chemical transformation from cobalt to cobalt phosphide nanoparticles. J. Mater. Chem. 2011, 21, 11498–11510.
- Teng, X.; Black, D.; Watkins, N.J.; Gao, Y.; Yang, H. Platinum-Maghemite Core−Shell Nanoparticles Using a Sequential Synthesis. Nano Lett. 2003, 3, 261–264.
- Yoon, T.J.; Lee, H.; Shao, H.; Weissleder, R. Highly magnetic core-shell nanoparticles with a unique magnetization mechanism. Angew. Chemie-Int. Ed. 2011, 50, 4663–4666.
- Zeng, H.; Li, J.; Wang, Z.L.; Liu, J.P.; Sun, S. Bimagnetic Core/Shell FePt/Fe3O4. Nanoparticles 2004, 4, 187–190.
- Zhou, T.; Lu, M.; Zhang, Z.; Gong, H.; Chin, W.S.; Liu, B. Synthesis and characterization of multifunctional FePt/ ZnO core/Shell nanoparticles. Adv. Mater. 2010, 22, 403–406.
- Cho, N.H.; Cheong, T.C.; Min, J.H.; Wu, J.H.; Lee, S.J.; Kim, D.; Yang, J.S.; Kim, S.; Kim, Y.K.; Seong, S.Y. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat. Nanotechnol. 2011, 6, 675–682.
- Kim, H.; Achermann, M.; Balet, L.P.; Hollingsworth, J.A.; Klimov, V.I. Synthesis and characterization of Co/CdSe core/shell nanocomposites: Bifunctional magnetic-optical nanocrystals. J. Am. Chem. Soc. 2005, 127, 544–546.
- Lee, J.-S.; Bodnarchuk, M.I.; Shevchenko, E.V.; Talapin, D.V. “Magnet-in-the-Semiconductor” FePt−PbS and FePt−PbSe Nanostructures: Magnetic Properties, Charge Transport, and Magnetoresistance. J. Am. Chem. Soc. 2010, 132, 6382–6391.
- Tian, Q.; Hu, J.; Zhu, Y.; Zou, R.; Chen, Z.; Yang, S.; Li, R.; Su, Q.; Han, Y.; Liu, X. Sub-10 nm Fe3O4@Cu2−xS Core−Shell Nanoparticles for Dual-Modal.pdf. J. Am. Chem. Soc. 2013, 135, 8571–8577.
- Xu, Z.; Hou, Y.; Sun, S. Magnetic core/shell Fe3O4/Au and Fe3O 4/Au/Ag nanoparticles with tunable plasmonic properties. J. Am. Chem. Soc. 2007, 129, 8698–8699.
- Wang, L.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.; Kim, N.; Wang, J.Q.; Zhong, C.J. Monodispersed core-shell Fe 3O nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601.
- Shi, W.; Zeng, H.; Sahoo, Y.; Ohulchanskyy, T.Y.; Ding, Y.; Wang, Z.L.; Swihart, M.; Prasad, P.N. A General Approach to Binary and Ternary Hybrid Nanocrystals. Nano Lett. 2006, 6, 875–881.
- Lu, Z.; Yin, Y. Colloidal nanoparticle clusters: Functional materials by design. Chem. Soc. Rev. 2012, 41, 6874–6887.
- Guimarães, T.R.; Lansalot, M.; Bourgeat-Lami, E. Polymer-encapsulation of iron oxide clusters using macroRAFT block copolymers as stabilizers: Tuning of the particle morphology and surface functionalization. J. Mater. Chem. B 2020, 8, 4917–4929.
- Li, Y.; Wang, N.; Huang, X.; Li, F.; Davis, T.P.; Qiao, R.; Ling, D. Polymer-Assisted Magnetic Nanoparticle Assemblies for Biomedical Applications. ACS Appl. Bio Mater. 2020, 3, 121–142.
- Tadic, M.; Kralj, S.; Kopanja, L. Synthesis, particle shape characterization, magnetic properties and surface modification of superparamagnetic iron oxide nanochains. Mater. Charact. 2019, 148, 123–133.
- Storozhuk, L.; Besenhard, M.O.; Mourdikoudis, S.; LaGrow, A.P.; Lees, M.R.; Tung, L.D.; Gavriilidis, A.; Thanh, N.T.K. Stable Iron Oxide Nanoflowers with Exceptional Magnetic Heating Efficiency: Simple and Fast Polyol Synthesis. ACS Appl. Mater. Interfaces 2021, 13, 45870–45880.
- Furrer, A. Magnetic cluster excitations. J. Phys. Conf. Ser. 2011, 325, 012001.
- Kratz, H.; Taupitz, M.; De Schellenberger, A.A.; Kosch, O.; Eberbeck, D.; Wagner, S.; Trahms, L.; Hamm, B.; Schnorr, J. Novel magnetic multicore nanoparticles designed for MPI and other biomedical applications: From synthesis to first in vivo studies. PLoS ONE 2018, 13, e0190214.
- Hobson, N.J.; Weng, X.; Siow, B.; Veiga, C.; Ashford, M.; Thanh, N.T.K.; Schätzlein, A.G.; Uchegbu, I.F. Clustering superparamagnetic iron oxide nanoparticles produces organ-Targeted high-contrast magnetic resonance images. Nanomedicine 2019, 14, 1135–1152.
- Hennion, M.; Pardi, L. Neutron study of mesoscopic magnetic clusters. Phys. Rev. B-Condens. Matter Mater. Phys. 1997, 56, 8819–8827.
- Narayanaswamy, A.; Xu, H.; Pradhan, N.; Peng, X. Crystalline nanoflowers with different chemical compositions and physical properties grown by limited ligand protection. Angew. Chemie-Int. Ed. 2006, 45, 5361–5364.
- Ge, J.; Hu, Y.; Biasini, M.; Beyermann, W.P.; Yin, Y. Superparamagnetic magnetite colloidal nanocrystal clusters. Angew. Chemie-Int. Ed. 2007, 46, 4342–4345.
- Dan, M.; Scott, D.F.; Hardy, P.A.; Wydra, R.J.; Hilt, J.Z.; Yokel, R.A.; Bae, Y. Block copolymer cross-linked nanoassemblies improve particle stability and biocompatibility of superparamagnetic iron oxide nanoparticles. Pharm. Res. 2013, 30, 552–561.
- Bernad, S.I.; Craciunescu, I.; Sandhu, G.S.; Dragomir-Daescu, D.; Tombacz, E.; Vekas, L.; Turcu, R. Fluid targeted delivery of functionalized magnetoresponsive nanocomposite particles to a ferromagnetic stent. J. Magn. Magn. Mater. 2021, 519, 167489.
- Turcu, R.; Craciunescu, I.; Garamus, V.M.; Janko, C.; Lyer, S.; Tietze, R.; Alexiou, C.; Vekas, L. Magnetic microgels for drug targeting applications: Physical-chemical properties and cytotoxicity evaluation. J. Magn. Magn. Mater. 2015, 380, 307–314.
- Larsen, B.A.; Haag, M.A.; Serkova, N.J.; Shroyer, K.R.; Stoldt, C.R. Controlled aggregation of superparamagnetic iron oxide nanoparticles for the development of molecular magnetic resonance imaging probes. Nanotechnology 2008, 19, 265102.
- Lim, E.K.; Jang, E.; Kim, B.; Choi, J.; Lee, K.; Suh, J.S.; Huh, Y.M.; Haam, S. Dextran-coated magnetic nanoclusters as highly sensitive contrast agents for magnetic resonance imaging of inflammatory macrophages. J. Mater. Chem. 2011, 21, 12473–12478.
- Craciunescu, I.; Petran, A.; Daia, C.; Marinica, O.; Vekas, L.; Turcu, R. Stimuli responsive magnetic nanogels for biomedical application. AIP Conf. Proc. 2013, 1565, 203–207.
- Kostopoulou, A.; Lappas, A. Colloidal magnetic nanocrystal clusters: Variable length-scale interaction mechanisms, synergetic functionalities and technological advantages. Nanotechnol. Rev. 2015, 4, 595–624.
- Lu, Z.; Duan, J.; He, L.; Hu, Y.; Yin, Y. Mesoporous TiO2 nanocrystal clusters for selective enrichment of phosphopeptides. Anal. Chem. 2010, 82, 7249–7258.
- Li, P.; Peng, Q.; Li, Y. Dual-Mode luminescent colloidal spheres from monodisperse rare-earth fluoride nanocrystals. Adv. Mater. 2009, 21, 1945–1948.
- Chen, C.; Nan, C.; Wang, D.; Su, Q.; Duan, H.; Liu, X.; Zhang, L.; Chu, D.; Song, W.; Peng, Q.; et al. Mesoporous multicomponent nanocomposite colloidal spheres: Ideal high-temperature stable model catalysts. Angew. Chemie-Int. Ed. 2011, 50, 3725–3729.
- Xu, F.; Cheng, C.; Xu, F.; Zhang, C.; Xu, H.; Xie, X.; Yin, D.; Gu, H. Superparamagnetic magnetite nanocrystal clusters: A sensitive tool for MR cellular imaging. Nanotechnology 2009, 20, 405102.
- Craciunescu, I.; Chiţanu, E.; Codescu, M.M.; Iacob, N.; Kuncser, A.; Kuncser, V.; Socoliuc, V.; Susan-Resiga, D.; Bălănean, F.; Ispas, G.; et al. High performance magnetorheological fluids: Very high magnetization FeCo–Fe3O4 nanoclusters in a ferrofluid carrier. Soft Matter 2022, 18, 626–639.
More
