Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Bertrand Liagre and Version 3 by Hicham Wahnou.
Prostate cancer is a major health concern worldwide, and current treatments, such as surgery, radiation therapy, and chemotherapy, are associated with significant side effects and limitations. Photodynamic therapy (PDT) is a promising alternative that has the potential to provide a minimally invasive and highly targeted approach to treating prostate cancer. PDT involves the use of photosensitizers (PSs) that are activated by light to produce reactive oxygen species (ROS), which can induce tumor cell death.
- photodynamic therapy
- prostate cancer
- reactive oxygen species
- cell death
1. Mechanism of Action
PDT uses a photosensitizer (PS), molecular oxygen, and light to generate reactive oxygen species (ROS) that can trigger tumor cell death [1][11]. PDT can cause tumor destruction by three mechanisms: direct cell death, tumor vascular damage, and an immune response (Figure 1) [1][2][11,12].
Direct cell death (Figure 1A) occurs through both programmed cell death (apoptosis) and non-programmed cell death (necrosis) pathways [3][13]. Necrosis is characterized by rapid cell death leading to the release of cellular components and molecules that promote inflammation, while apoptosis is a genetically encoded, energy-dependent process that can initiate an immune response [4][5][14,15]. PDT can also induce unconventional modes of cell death in cancer cells, including paraptosis, parthanatos, mitotic catastrophe, pyroptosis, necroptosis, and ferroptosis [3][13]. In addition, a growing body of evidence suggests that PDT can also trigger immunogenic cell death (ICD) [6][16], which has emerged as a promising strategy for eliminating tumor cells by promoting T-cell adaptive immune responses and inducing durable immunological memory [3][6][7][13,16,17].
PDT can also damage the tumor microvasculature (Figure 1B), leading to the interruption of the tumor’s feeding and, consequently, to the death of the tumor cells. This vascular mechanism is achieved by concentrating the PS in the vascular system and using a short drug-light interval [8][18]. PDT vascular effect can be selectively applied to the tumor and surrounding healthy tissue, with important advantages over PDT protocols that require PS accumulation in the tumor cells [7][8][17,18].
Finally, PDT can induce an immune response (Figure 1C) that can contribute to long-term tumor control [8][18]. PDT has been shown to influence the adaptive immune response through either stimulation or suppression, depending on the treatment protocol [9][6]. The oxidative damage inflicted by PDT on the tumor stroma triggers an acute inflammatory response initiated by the release of pro-inflammatory mediators. These mediators attract the host’s innate immune cells, which can activate a systemic antitumor immune response. PDT-induced necrosis of tumors and their vasculature can activate CD8 cytotoxic T lymphocytes that can specifically destroy tumor cells and circulate throughout the body for long periods [8][18].
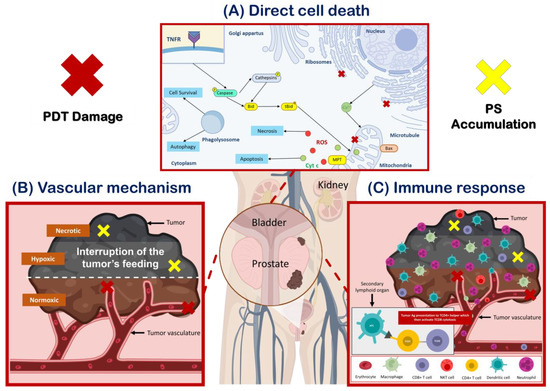
Figure 1. PDT destroys tumor cells by three mechanisms of action. (A) Induction of cell death through various pathways depends on the location of the photosensitizer (PS) and the extent of damage to the organelles. Mitochondrial damage caused by PDT results in the loss of membrane permeability and the release of pro-apoptotic mediators, while damage to the endoplasmic reticulum (ER) leads to the release of cellular calcium deposits. Lysosomal damage, on the other hand, causes the release of proteolytic enzymes upon illumination, and can also trigger autophagy. In cases where apoptosis is dysfunctional, necrosis and autophagy may be the primary modes of cell death after PDT. It is important to note that multiple PSs may localize in multiple organelles, and therefore, simultaneous activation of death pathways may occur (adapted from Mroz, P et al. [10][19]). (B) PDT can induce vascular damage, leading to the interruption of the tumor’s feeding causing its death through various mechanisms. (C) PDT can also activate innate and adaptative immunity, leading to a systemic antitumor immune response and the destruction of tumor cells.
2. Photosensitizers (PSs)
PSs are molecules that can absorb light and transfer the absorbed energy to the surrounding molecules (e.g., oxygen), leading to the generation of reactive oxygen species (ROS) that can cause cell death. These PSs are generally divided into two categories: tetrapyrrolic macrocycles (i.e., porphyrins, chlorins, bacteriochlorins, phthalocyanines, and their derivates) and no tetrapyrrolic macrocycles (i.e., methylene blue, curcumin, phenalenon, coumarin, anthraquinone, boron-dipyrromethene florophore (bodipy), and their derivatives) [11][12][13][14][20,21,22,23]. Tetrapyrrolic macrocycles are the most widely used PSs in anticancer PDT and, among them, porphyrins are the most studied. Thus, porphyrin-derived PSs are further classified into three generations [15][24].
The first-generation PS, such as the hematoporphyrin derivative (HpD) and Photofrin®, were the first photosensitizers to be used clinically in PDT. However, these molecules have some drawbacks, such as a mixture of PSs, prolonged skin photosensitization, no selectivity, and suboptimal tissue penetration [16][25].
Second-generation PSs were developed to address these issues. These compounds are chemically pure, absorb light at a longer wavelength, and cause significantly less skin photosensitization post-treatment. They must also be at least as efficient in eradicating tumors as Photofrin®, which is currently the gold standard for PDT [17][26].
Third-generation PSs, which are bound to targeting agents (antibodies, sugar, folic acid, polyamine, peptide, etc.), are able to be selectively accumulated within tumor tissues and represent an active research area in the field [18][19][20][21][22][23][24][25][26][27,28,29,30,31,32,33,34,35].
Of note, fourth-generation PSs are still in the early stages of development and are characterized by their ability to be associated with nanoparticles and/or activated by specific stimuli, such as light at a specific wavelength, changes in pH or temperature, ultrasound, or X-rays [27][36]. This allows for even greater control over the location and timing of the PS activation, potentially reducing side effects and improving treatment efficacy. Fourth-generation PSs are still a relatively new area of research but have shown promising results in preclinical studies [28][29][30][31][37,38,39,40].
3. Natural and Synthetic PS
Herbal medicines have been used for centuries to treat various human ailments, and many modern medicines are derived from medicinal plants. There is a growing interest in using natural products, including herbal extracts and compounds, as possible lead components in cancer drug discoveries. Natural products are a valuable source for the development of drugs and the discovery of new PSs [32][33][34][35][41,42,43,44]. In recent decades, efforts have been made to isolate new products of natural origin from microbes, plants, and other living organisms, leading to the discovery of a number of anti-cancer drugs [32][41]. Researchers have found new active chemical compounds in natural extracts that display efficient PS properties, and numerous compounds have already been identified as potential candidates to be used as PSs [35][44]. Furthermore, screening studies of natural extracts have identified new PSs with cyclic tetrapyrrole structures [36][45]. Additionally, in a 2022 review published by Kubrak TP et al., various photochemicals were described for their light-absorbing properties and their anticancer activity including furanocoumarins, polyacetylenes, thiophenes, curcumins, alkaloids, and anthraquinones [11][20].
On the other hand, synthetic PSs are those that are created in the laboratory through chemical synthesis. These PSs are designed to have specific properties that allow them to effectively target cancer cells and destroy them upon exposure to light. They are often optimized to have higher light absorption at longer wavelengths, improved tissue penetration, and reduced toxicity compared to earlier generations of PSs [28][37][37,46]. Synthetic PSs can be produced in large quantities and with a high degree of purity, making them a reliable and cost-effective option for PDT. Some examples of synthetic PSs include benzoporphyrins, texaphyrins, purpurin, chlorin e6, bacteriochlorin. and pyropheophorbide.
Figure 2 presents some natural and synthetic PSs according to their absorption wavelengths.
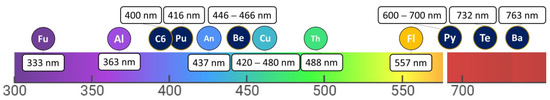
Figure 2. Absorption wavelengths of non-tetrapyrrolic PS derivatives (furanocoumarins (Fu), thiophenes (Th), curcumins (Cu), flavonoids (Fl), anthraquinones (An), alkaloids (Al)) and tetrapyrrolic PS derivatives (benzoporphyrin (Be), texaphyrins (Te), purpurin (Pu), chlorin e6 (C6), bacteriochlorin (Ba), pyropheophorbide (Py)).
An updated summary of natural and synthetic PSs and their potential mechanism of action to target prostate cancer are presented in Figure 3.
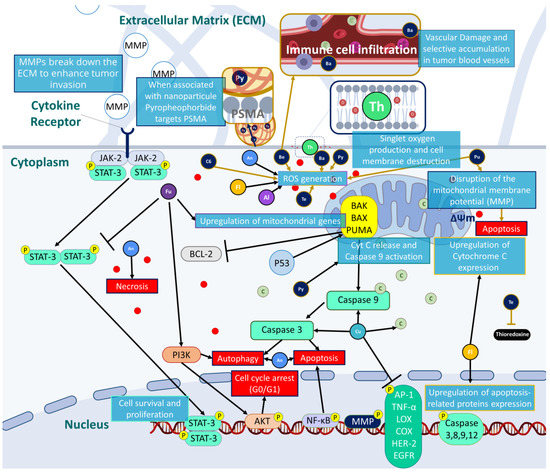
Figure 3. Possible mechanisms of action to induce prostate cancer eradication by targeting several pathways involving oxidative stress, cell death and cell cycle arrest.
4. Ongoing Clinical Studies in Prostate Cancer
In clinical settings, the implementation of PDT for prostate cancer involves several key steps. First, a photosensitizing agent is administered to the patient, either systemically or directly into the tumor site. The choice of photosensitizer depends on factors such as its absorption and distribution properties, as well as its ability to selectively accumulate in tumor tissues [38][74]. Once the photosensitizer has been adequately distributed, a specific light source is selected, considering factors, such as wavelength and power output [38][74]. Light delivery techniques may vary, including intraluminal or interstitial illumination [39][75]. For instance, intraluminal light sources can be used for prostate cancer by inserting light-emitting devices through catheters or endoscopes. Interstitial PDT involves the placement of optical fibers or diffusers directly into the prostate tissue, allowing for light delivery to deeper tissue layers [39][75]. These techniques aim to achieve optimal light distribution and tumor coverage.
PDT is a promising treatment option for prostate cancer, with the potential to provide a safe and effective alternative to conventional therapies. However, in order to fully evaluate the safety and efficacy of PDT for prostate cancer, clinical trials are needed. These trials can provide valuable insights into the effectiveness of PDT, as well as help to identify any potential side effects or limitations of the treatment.
To date, several clinical trials have been conducted on the use of PDT for prostate cancer, with promising results.
5. Multimodal Synergistic Therapies to Overcome PDT Limitations
Combination approaches in PDT for cancer treatment address limitations related to tumor size and location [40][78]. Larger tumors and tumors located in challenging anatomical areas can pose difficulties in achieving complete eradication with PDT alone due to the limited light penetration depth [41][79]. However, by combining PDT with other treatment modalities, such as surgery, sonodynamic therapy, photoimmunotherapy, chemotherapy, and photothermal therapy, these limitations can be overcome [42][80]. For larger tumors, combination approaches can sensitize the tumor or target residual cancer cells, thus enhancing the therapeutic effect. Multimodal therapies that combine PDT with other treatments have shown promise in the treatment of multidrug-resistant and hypoxia-related prostate cancers (Figure 4) [43][44][4,81]. However, challenges arise in terms of complexity, potential side effects and the requirement for rigorous testing of optimal sequencing and timing strategies.
5.1. PDT/Surgery
Surgery is a common treatment for prostate cancer, but it is not always successful in eradicating all cancer cells. Recent studies have explored the potential benefits of combining surgery with PDT in the treatment of prostate cancer. A retrospective study published in 2019 by Pierrard V et al. [45][82] investigated the safety and effectiveness of salvage radical prostatectomy (surgical removal of the prostate gland) after vascular-targeted photodynamic therapy (VTP) treatment. The study included 45 patients who underwent a salvage prostatectomy followed by VTP salvage due to persistent or recurrent cancer. The results showed that the combination was a safe and effective treatment option for localized prostate cancer. The study also reported that the combination therapy did not result in any severe complications or adverse events [45][82]. Furthermore, the feasibility, safety, and effectiveness of this combination in treating most locally recurrent prostate cancer were also reported by a previous study where various outcomes were reported including operation time, blood loss, complications, urethral catheterization time, functional outcomes, and short-term oncologic outcomes [46][83].
5.2. PDT/Sonodynamic Therapy
Although there are no specific studies on the use of PDT and sonodynamic therapy (SDT) for prostate cancer treatment, these therapies are currently being studied for their potential in cancer treatments [30][47][39,84]. PDT and SDT utilize sensitizers that can produce ROS, which can cause damage to cancer cell membranes. The combination of these therapies has demonstrated promising results in preclinical studies for various types of cancer, such as brain cancer [30][39]. In fact, in the prostate cancer context, the penetrating power of ultrasound avoids the use of an endoscope (and light) in the bladder while allowing the activation of the PS [47][84]. Ongoing research aims to identify effective sensitizers and optimal delivery methods to enhance the effectiveness of PDT and SDT in prostate cancer treatments. While further studies are necessary to determine the safety and efficacy of these therapies for prostate cancer, the potential benefits of PDT and SDT offer a promising avenue for the development of effective cancer treatments.
5.3. PDT/Photoimmunotherapy
Photoimmunotherapy (PIT) is an innovative treatment that harnesses the power of phototherapy and immunotherapy to combat cancer by inducing antitumor immune responses and preventing cancer recurrence [48][85]. Among the different approaches to PIT, one of the most promising and rapidly expanding is near-infrared (NIR)-PIT. This approach selectively and locally destroys cancer cells by releasing damage-associated molecular patterns (DAMPs) and tumor-associated antigens (TAAs), which trigger an antitumor immune response. NIR-PIT has demonstrated remarkable results in preclinical and clinical applications for various types of cancer including prostate cancer [48][49][85,86]. In fact, in a study using a prostate-specific membrane antigen (PSMA)-expressing a prostate cancer cell line, NIR-PIT with a fully human IgG1 anti-PSMA monoclonal antibody (mAb) conjugated to a specific photoabsorber resulted in specific binding and cell-specific killing after exposure to NIR light [49][86].
5.4. PDT/Chemotherapy
In the last few years, researchers have developed a new treatment strategy for prostate cancer known as photochemotherapy. This approach combines the benefits of PDT and chemotherapy to deliver cancer-fighting drugs selectively to tumor cells. Unlike traditional chemotherapy, photochemotherapy can target cancerous tissues without damaging surrounding healthy cells, potentially reducing unwanted side effects. By combining these two therapies, the effectiveness of the treatment is enhanced, with chemotherapy compensating for the limited light penetration in the PDT. Furthermore, chemotherapy may increase the sensitivity of cancer cells to ROS or hyperthermia, resulting in a synergistic effect [50][87]. Furthermore, in vivo studies using athymic nude mice and BALB/c nude mice, have also demonstrated the effectiveness of photochemotherapy in treating prostate cancer [51][52][88,89].
5.5. PDT/Photothermal Therapy
Photothermal therapy (PTT) is a form of phototherapy that uses light energy to produce heat energy, which can kill cancer cells [43][4]. PTT has been used to treat prostate cancer by irradiating a photothermal agent with an external light source of a specific wavelength, typically NIR light. PTT has been combined with PDT to enhance the effects of both therapies. The thermal effect of PTT can improve local blood flow and increase the oxygen concentration in the tumor tissue, making up for the deficiency of PDT in a hypoxic environment [53][90]. Many studies have reported the combination of PDT and PTT using nanomaterials as drug carriers [43][53][4,90]. Some studies have used self-assembled nanoparticles, such as melanin-like polydopamine nanoparticles, to deliver photothermal and photodynamic agents for synergistic therapy [43][53][4,90]. However, the low photoconversion efficiency of some materials used in these therapies raises issues about how to maximize the synergistic effect of PDT and PTT.
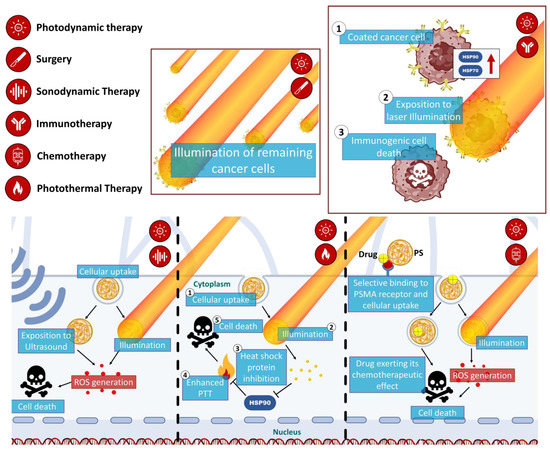
Figure 4. Schematic illustration of mechanisms of the multimodal synergistic therapies targeting prostate cancer using PDT.
6. Conclusion
In conclusion, Photodynamic Therapy (PDT) holds great promise as a minimally invasive and highly targeted approach for treating prostate cancer, with minimal side effects on healthy tissues. PDT has gained regulatory approval, and its combination with other therapeutic modalities shows potential against tumor growth. Ongoing research is focused on enhancing PDT through innovative organic photosensitizers and drug delivery systems. Moreover, PDT has demonstrated effectiveness in treating various cancer types and non-cancer conditions. Despite significant progress, further clinical studies are needed to refine PDT modalities and improve its overall efficacy
Schematic illustration of mechanisms of the multimodal synergistic therapies targeting prostate cancer using PDT.
