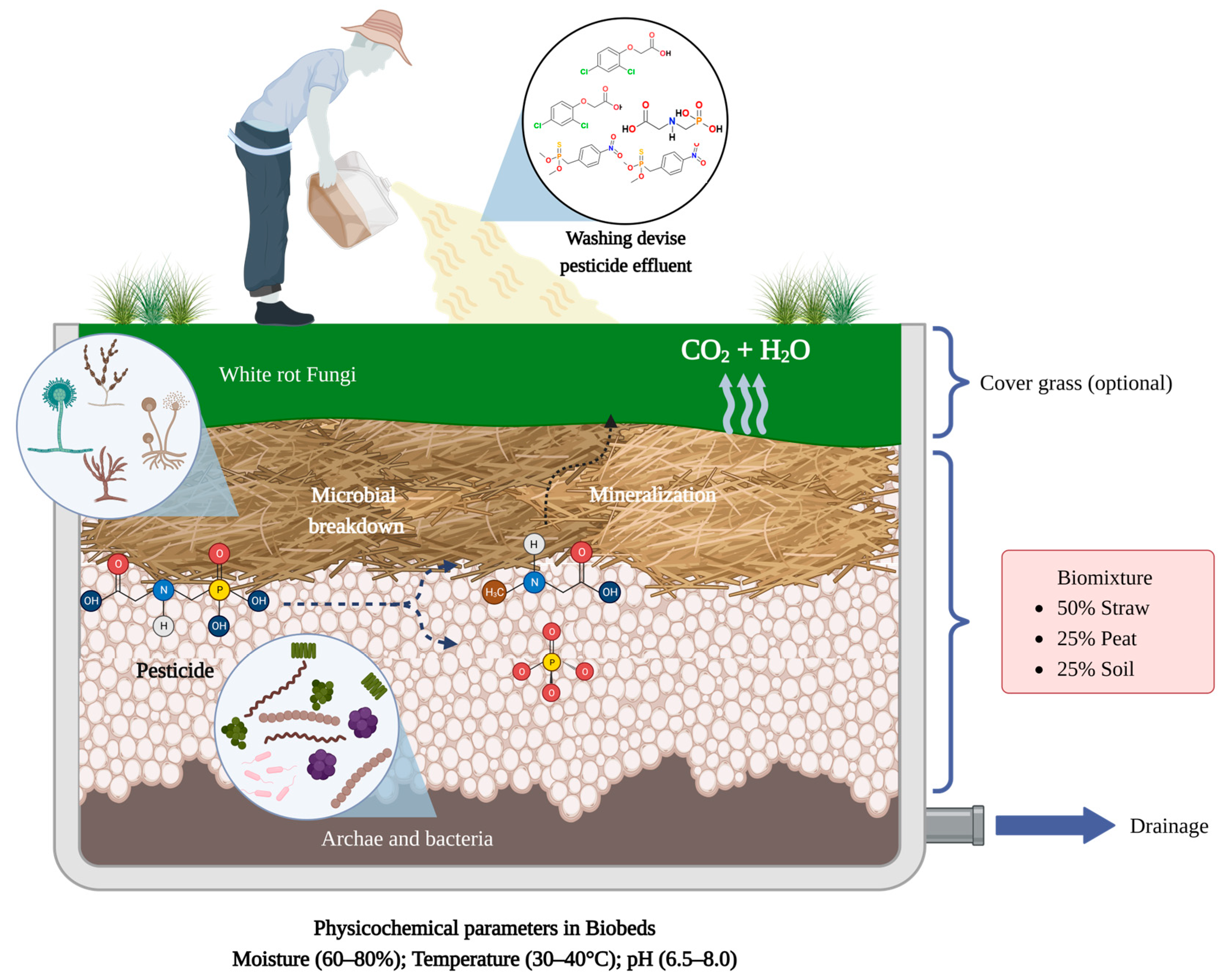
Biobeds are biological systems used to treat liquid residues derived from the operations related to the application of pesticides in crop fields. Their use helps minimize pesticide delivery into the environment, as well as protecting soil and water from pollution. Biobeds were first described as trenches packed with a mixture of 50% wheat straw, 25% soil, and 25% peat, covered with a grass layer; this composition is known as a “biomixture”. In biobeds, the biomixture absorbs the pesticide residues and supports the development of different microorganisms, such as bacteria and fungi, needed for pesticide degradation in the system. The effectiveness of biobed systems lies in the high pesticide retention in the biomixture and the degradation potential of the microorganisms growing in the system.
- biodegradation
- fungicides
- herbicides
- microorganisms
- pesticide residues
- insecticides
1. Introduction
2. Release of Pesticide Residues into the Environment
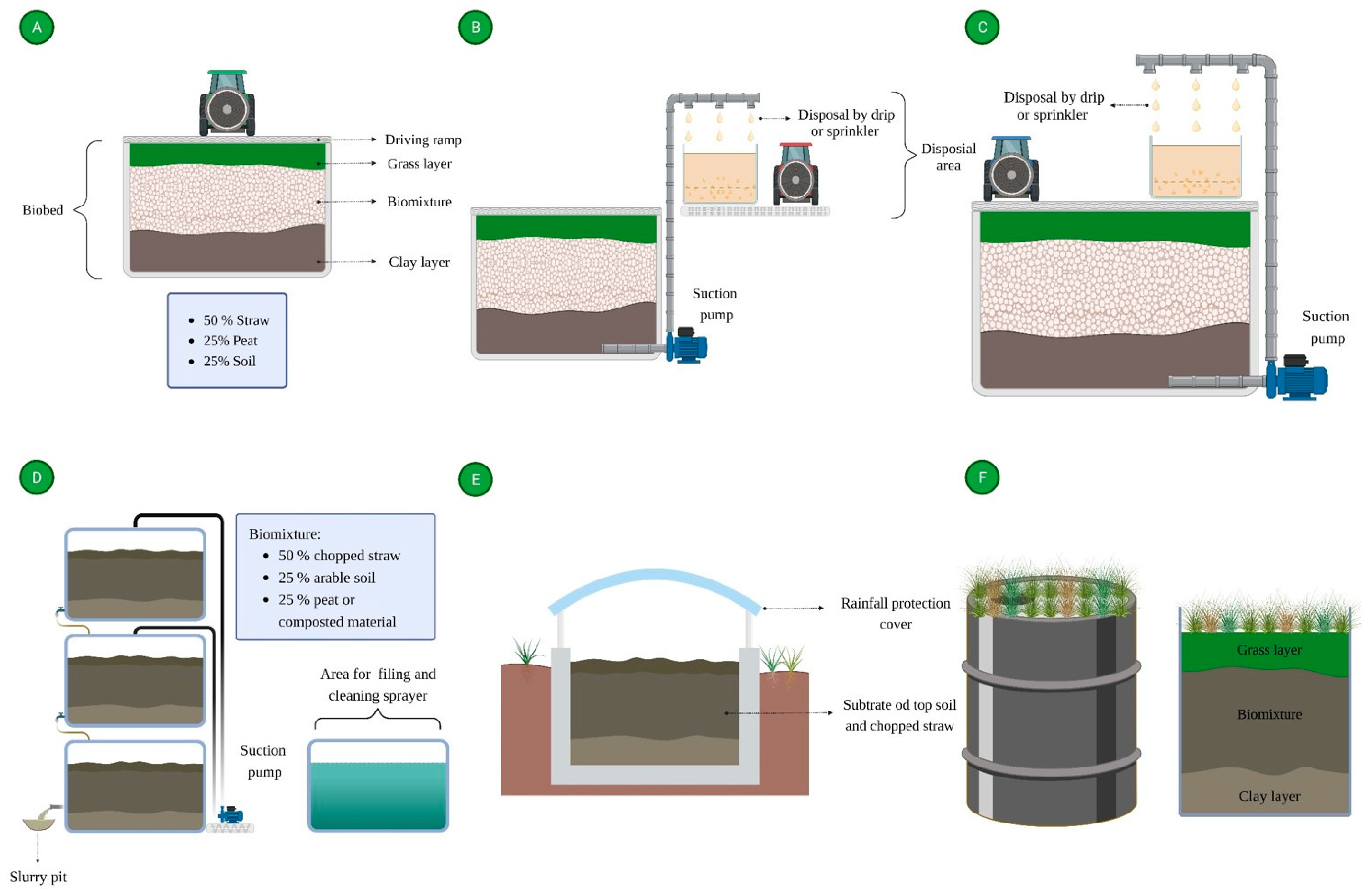
3. What Are Biobeds?
4. Key Factors in Biobeds’ Effectiveness
4.1. Biomixture
The composition of the biomixture is a key factor for the efficiency of pesticide degradation in biobed systems; so, each component (wheat straw, soil, and peat) plays an important role [62]. For example, wheat straw is a lignocellulosic substrate that acts as an adsorbent for pesticides in the system, serves as physical support for the development of microbial communities, provides essential nutrients for the growth of fungi and bacteria, and stimulates the production of ligninolytic enzymes, such as laccases and peroxidases, reported to be highly efficient in the degradation of different pesticides. The soil supplies microorganisms to the system and stimulates the microbial activity that mediates the degradation of the pesticides, while peat is a porous material that increases pesticide retention in the biobed system, regulates the moisture, and reduces the pH, factors that favor pesticide dissipation [63,64,65,66][63][64][65][66]. In the biomixture, wheat straw can be replaced by other lignocellulosic substrates, depending on the availability of these materials in a particular country where biobed systems are applied. For example, Karanasios et al. (2010) [67] reported the use of different low-cost lignocellulosic materials, such as sunflower crop residues, olive leaves, grape stalks, orange peels, corn cobs, and spent mushroom substrate for the degradation of mixtures of pesticides in biobed systems. In this study, the alternative substrates favored the retention of pesticides in the system, and comparable pesticide half-life values, concerning those observed in the biobeds with the presence of wheat straw, were documented. In another study, Diez et al. (2013) [68] complemented the biomixture composition with the addition of lignocellulosic materials, such as pine sawdust (25%) and barley husk (25%), for the degradation of the pesticides carbendazim, isoproturon, and chlorpyrifos. The systems that contained wheat straw/barley husk (25%/25%) showed higher degradation percentages for carbendazim and chlorpyrifos after 90 days compared to the systems with only wheat straw (50%) and wheat straw/pine sawdust (25%/25%). In a similar study, Urrutia et al. (2013) [66] evaluated the addition of lignocellulosic materials such as barley husk, oat husk, and sawdust to biobed biomixtures for the treatment of the pesticides atrazine, chlorpyrifos, and isoproturon. Among the three lignocellulosic materials, oat husk was the best substitute for wheat straw, with similar pesticide degradation rates compared to the biomixture that included just wheat straw. In contrast, barley husk and sawdust can be added to the biomixtures in combination with wheat straw but not as the sole lignocellulosic material in the biomixture composition. Gongora-Echeverría et al. (2017) [69] evaluated the suitability of wheat straw substitution in biobed systems, employing different materials of high availability in southeastern Mexico such as compost, sisal fibers, corn stoves, and seaweed in combination with soil for the treatment of a pesticide mixture composed of 2,4-dichloro phenoxy acetic acid (2,4-D, 1.08 mg/cm3 of mixture), atrazine (2.5 mg/cm3 of mixture), carbofuran (0.23 mg/cm3 of mixture), diazinon (0.34 mg/cm3 of mixture), and glyphosate (0.36 mg/cm3 of mixture), mimicking the composition of pesticide effluents generated by farmers in Yucatan, Mexico. In all evaluated biomixtures, the five pesticides’ dissipation was over 99% after 41 days. Peat is an important component in biobed biomixtures; however, in some regions, this material has low availability or high cost. So, in biomixtures, peat has been substituted by alternative material or just eliminated from the biomixture composition [70,71][70][71]. Among the alternative materials to peat for biobed mixtures, compost [72,73,74,75,76][72][73][74][75][76] or vermicompost [43,77,78,79][43][77][78][79] are notable for being the most reported. Various agro-industrial wastes have been employed in the biomixture composition in biobed systems to treat fungicides, herbicides, and insecticides from different chemical families. They include spent coffee grounds [80], coir [80], cotton crop residues [81[81][82],82], garden wastes [83[83][84],84], livestock manure [80[80][85],85], olive leaves [68[68][86][87],86,87], pine bark [80], and sewage sludge [88]. Spent mushroom substrates and biochar have also been incorporated into the biomixture composition as complementary materials in pesticide dissipation [38,39,42,70,89,90,91][38][39][42][70][89][90][91].4.2. Microorganisms
In the biobed systems, the microbiota colonizing the biomixture are responsible for the pesticide degradation. Microorganisms such as bacteria and fungi may use pesticide molecules such as carbon, nitrogen, phosphorous, and energy sources for their growth. The efficient pesticide degradation by microorganisms is related to their great genetic plasticity, the production of diverse pesticide-degrading enzymes, fast growth, and adaptability to living in polluted environments [89]. The materials that integrate the biomixture retain the pesticide molecules in the biobeds and serve as a habitat for the development of different soil autochthonous microorganisms [92]. In biobeds, the presence of lignocellulosic materials reduces the pH in the system generating an environment that favors the growth and development of lignin-degrading fungi, such as different species of white-rot fungi [45]. White-rot fungi are organisms broadly reported in the biodegradation of several organic pollutants, including pesticides from different chemical families [93,94,95,96,97,98][93][94][95][96][97][98]. Fungi can produce extracellular enzymes, such as peroxidases, laccases, and the cytochrome P450 complex, implicated in pesticide degradation [99,100,101][99][100][101]. On the other hand, the presence of peat in the biomixture also favors the development of white-rot fungi in biobeds; however, in biomixtures without peat, the pesticide degradation is mediated mainly by the bacterial community [45]. Bacteria may act in synergy with fungi to enhance the pesticide and derived metabolites degradation; so, they can also produce different pesticide-degrading enzymes, such as dehalogenases, hydrolases, oxidoreductases, oxygenases, and esterases [102,103,104,105,106][102][103][104][105][106]. In biobed systems, the biomixture supports the development of broad microbial diversity, and recent studies have evaluated such microbial complexity. For example, through a metagenomics approach, Bergsveinson et al. (2018) [107] assessed the bacterial and fungal diversity in four biobed systems employed for treating pesticide rinsates with differential composition and pesticide concentrations. As a result of the study, around 440 bacterial genera and an average of 285 fungal genera were identified in each biobed system. In a similar study, Góngora-Echeverría et al. (2018) [108] identified several archaea (23), bacteria (598), and fungi (64) species in lab-scale biobed systems with the presence of a mixture of commercial pesticide formulates (2,4-D, atrazine, carbofuran, diazinon, and glyphosate). In addition, Russell et al. (2021) [109] evaluated the bacterial diversity in a two-cell biobed system. After the treatment of pesticide residues, in cell one, 81 bacterial species from 58 genera were identified, while in cell two, 36 bacterial species from 33 genera were identified. The most representative bacterial genera in both biobed cells were Afipia, Sphingopyxis, and Pseudomonas. The development of a great diversity of microorganisms in the biobed systems is crucial for the efficient treatment of pesticide residues. However, the metabolic activities of the indigenous microbiota do not always guarantee total pesticide degradation. Due to this, bioaugmentation strategies have been employed to enhance pesticide biodegradation efficiency in biobed systems. This strategy is based on the addition of selected endogenous or exogenous microorganisms, such as specific fungi and bacterial strains, or the use of characterized or non-characterized microbial consortia [45,52][45][52]. The key characteristics for selecting microorganisms for a bioaugmentation strategy include pesticide resistance, high pesticide degradation efficiency, fast growth, and simple culture in lab conditions [110,111][110][111]. Examples of microorganisms used in biobed bioaugmentation strategies include uncharacterized microbial consortia, archaea species, bacteria of different phyla such as Actinobacteria (Streptomyces spp.), Bacteroidetes, Firmicutes, and mainly Proteobacteria (Achromobacter ssp., Bordetella ssp., Pseudomonas ssp., and Variovorax ssp.), and white-rot fungi from different classes (Aphelidiomycetes and Pezizomycetes) and species (Trametes versicolor and Stereum hirsutum).4.3. Physicochemical Parameters
Pesticide dissipation effectiveness in biobed systems is strongly related to the biomixture composition and the metabolic activity of the different microorganisms; however, other key parameters include the pre-incubation time, moisture, temperature, and pesticide concentration in the system. Fernández-Alberti et al. (2012) [112] evaluated the effect of the biomixture pre-incubation time and moisture on chlorpyrifos (insecticide) degradation. In the study, the biomixture was composed of wheat straw, peat, and soil (2:1:1), pre-incubation took place (25 ± 1 °C) over 0, 15, and 30 days, and three water-holding-capacity percentages (WHC 40%, 60%, and 80%) were evaluated. The best condition for chlorpyrifos degradation (>70%) was 15 days pre-incubation and 60% WHC. Pre-incubation favors the microbial community proliferation in the biomixture, while at a high moisture (60% WHC), the ligninolytic enzyme activity in the biomixture increases. In a similar study, Tortella et al. (2012) [113] evaluated the effect of the biomixture maturity and concentration on the chlorpyrifos degradation; the biomixture (wheat straw, peat and soil, 2:1:1) was pre-incubated over 0, 15, and 30 days; after that time, three chlorpyrifos concentrations (200, 320, and 480 mg·kg−1) were added to the biomixture. The biomixture’s maturity did not affect the chlorpyrifos degradation; all the biomixtures showed degradation percentages above 50%. However, increasing the chlorpyrifos concentration reduced the degradation efficiency and the hydrolytic and phenoloxidase activities in the systems. More recently, Kumari et al. (2019) [114] evaluated the effect of pre-incubation, pesticide concentration, and moisture on the degradation process of azoxystrobin (fungicide) and imidacloprid (insecticide) in biobed systems, employing biomixtures that included rice straw/corn cobs, peat, and compost (2:1:1). Ten days of biomixture pre-incubation before pesticide application reduced by 5–9 times the degradation rate of the insecticide imidacloprid, while the increase in the WHC from 60% to 80% had a positive effect on the degradation rates of both pesticides, reducing their half-life time. However, increases in the concentration of the pesticides from 30 to 100 mg·kg−1 reduced the degradation rates of both pesticides. Cordova-Méndez et al. (2021) [46] evaluated the effect of moisture and temperature on the dissipation of five pesticides, two insecticides: carbofuran and diazinon, and three herbicides: atrazine, 2,4-D, and glyphosate; five temperatures (5, 15, 25, 35, and 45 °C) and five water holding capacity percentages (20%, 40%, 60%, 80%, and 100%) were evaluated. The increasing temperature positively affected the dissipation of the five pesticides evaluated; the highest dissipation percentages were observed at temperatures of 35 and 45 °C. However, the increase in the water-holding percentages did not show a significant improvement in pesticide dissipation. The observed increase in pesticide dissipation was related to higher microbial activity at higher temperatures. According to the reviewed studies, for efficient pesticide treatment in biobed systems, physicochemical parameters, such as the preincubation time, moisture, temperature, and pesticide concentration in the system, must be optimized.4.4. Analysis of the Biobeds’ Treated Effluents
According to the biobed design, the systems are isolated from the soil through an impermeable layer, and it has been proposed that treated effluents ending from biobed systems could be reused for crop irrigation [115]. However, leachate analysis in biobed systems is essential to guarantee the efficiency of the treatment process and avoid releasing pesticides into the environment, causing soil and water pollution. In this sense, Henriksen et al. (2003) [58] evaluated the dissipation of the herbicides mecoprop and isoproturon in a biobed system. To determine the pesticide dissipation, the concentration of both herbicides in the leachate was assessed after a year, the concentrations of isoproturon and mecoprop were of 1.4% and 13%, respectively, of the initial dose (8 g), and the presence of a higher concentration of mecoprop in the biobed leachate was associated with its lower retention in the biobed biomixture. The excessive effluent load in the biobed systems could be a limiting factor for pesticide retention and dissipation efficiency, causing the release of leachates with pesticide concentration above the limits established in the regulations. Foog et al. (2004) [59] evaluated the effect of reducing the effluent loads in a biobed system (1.5 m deep) over the concentration of pesticides in leachates. They observed that a reduction from 1175 to 688 L/m2 of biomixture decreased the pesticide concentration in leachate from <0.32% to <0.006%, while a decrease to 202 L/m2 reduced the pesticide concentration to <0.0001%; according to their results, the maximum water holding in the system for efficient pesticide dissipation was 1121 L/m2. In another study, Spliid et al. (2006) [116] evaluated the presence of pesticides in leachates from a biobed system. The concentrations of 21 pesticides (5 g each in the mixture) were assessed through LC-MS/MS; after 593 days of treatment, only the herbicide bentazone showed a significant presence in leachates (14% of the original dose), ten pesticides were not detected in leachates, and the ten remaining pesticides showed reductions below 2% of the initial dose. The authors concluded that the biobeds were effective in retaining and degrading pesticides, generating effluents with lower pesticide concentrations. More recently, Karas et al. (2015) [38] evaluated the risk associated with the environmental release of the biobed-depurated wastewater, the leachates containing fungicides from pilot biobed systems. The leachates included traces of fungicides (diphenylamine, imazalil, ortho-phenylphenol, and thiabendazole); acute effects were evaluated in aquatic organisms, such as the crustacean Daphnia magna and the fish Oncorhynchus mykiss, while chronic effects were assessed in the fish Oncorhynchus mykiss, the algae Pseudokirchneriella subcapitata, and sediment-dwelling invertebrates such as Chironomus sp. The biobed-depurated effluents with diphenylamine, imazalil, and ortho-phenylphenol did not show either an acute or chronic exposure risk in any bioindicator organism; only the effluents with thiabendazole showed an acute exposure risk for Daphnia magna and a chronic exposure risk for Oncorhynchus mykiss. In the same study, the treatment of fungicides using bioaugmentation with fungicide-degrader bacteria generated effluents that showed no acute or chronic exposure risk for the organisms evaluated. The authors conclude that biobed-treated effluents do not represent an environmental risk and can be safely disposed.References
- Pawlak, K.; Kołodziejczak, M. The role of agriculture in ensuring food security in developing countries: Considerations in the context of the problem of sustainable food production. Sustainability 2020, 12, 5488.
- Pereira, P.; Barceló, D.; Panagos, P. Soil and water threats in a changing environment. Environ. Res. 2020, 186, 109501.
- Maja, M.M.; Ayano, S.F. The impact of population growth on natural resources and farmers’ capacity to adapt to climate change in low-income countries. Earth Syst. Environ. 2021, 5, 271–283.
- Molajou, A.; Afshar, A.; Khosravi, M.; Soleimanian, E.; Vahabzadeh, M.; Variani, H.A. A new paradigm of water, food, and energy nexus. Environ. Sci. Pollut. Res. 2021.
- Gomes, H.D.O.; Menezes, J.M.C.; da Costa, J.G.M.; Coutinho, H.D.M.; Teixeira, R.N.P.; do Nascimento, R.F. A socio-environmental perspective on pesticide use and food production. Ecotoxicol. Environ. Saf. 2020, 197, 110627.
- Mandal, A.; Sarkar, B.; Mandal, S.; Vithanage, M.; Patra, A.K.; Manna, M.C. Impact of agrochemicals on soil health. In Agrochemicals Detection, Treatment and Remediation, Pesticides and Chemical Fertilizers; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 161–187.
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, history, and classification. In Natural Remedies for Pest, Disease and Weed Control; Chukwuebuka Egbuna, C., Sawicka, B., Eds.; Academic Press: Oxford, UK, 2020; pp. 29–42.
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 283, 124657.
- Maksymiv, I. Pesticides: Benefits and hazards. J. Vasyl Stefanyk Precarpath. Nat. Univ. 2015, 2, 70–76.
- Castrejón-Godínez, M.L.; Rodríguez, A.; Sánchez-Salinas, E.; Mussali-Galante, P.; Tovar-Sánchez, E.; Ortiz-Hernández, M.L. Soils Contaminated with Persistent Organic Pollutants (POPs): Current Situations, Management, and Bioremediation Techniques: A Mexican Case Study. In Pesticides Bioremediation; Siddiqui, S., Meghvansi, M.K., Chaudhary, K.K., Eds.; Springer: Cham, Switzerland, 2022; pp. 413–453.
- Brühl, C.A.; Zaller, J.G. Biodiversity decline as a consequence of an inappropriate environmental risk assessment of pesticides. Front. Environ. Sci. 2019, 7, 177.
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. In Agrochemicals Detection, Treatment and Remediation, Pesticides and Chemical Fertilizers; Agrochemicals detection, treatment and remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 25–54.
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446.
- Dhananjayan, V.; Jayanthi, P.; Jayakumar, S.; Ravichandran, B. Agrochemicals Impact on Ecosystem and Bio-monitoring. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer: Singapore, 2020; pp. 349–388.
- Phat, C.; Ty, B.; Kuok, F.; Andrews, E.M.; Kurniawan, W.; Hinode, H. Residual Pesticides. In Water and Life in Tonle Sap Lake; Yoshimura, C., Khanal, R., Sovannara, U., Eds.; Springer: Singapore, 2022; pp. 387–397.
- Shalaby, S.; Abdou, G. The influence of soil microorganisms and bio-or-organic fertilizers on dissipation of some pesticides in soil and potato tubers. J. Plant Prot. Res. 2010, 50, 86–92.
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112.
- Dollimore, L.; Schimpf, W. Obsolete pesticide stocks–the past 25 years, lessons learned and observations for the future. Outlooks Pest Manag. 2013, 24, 251–256.
- Vijgen, J.; Weber, R.; Lichtensteiger, W.; Schlumpf, M. The legacy of pesticides and POPs stockpiles—A threat to health and the environment. Environ. Sci. Pollut. Res. 2018, 25, 31793–31798.
- Alshemmari, H. Inventories and assessment of POPs in the State of Kuwait as a basis for Stockholm Convention implementation. Emerg. Contam. 2021, 7, 88–98.
- Mit, N.; Cherednichenko, O.; Mussayeva, A.; Khamdiyeva, O.; Amirgalieva, A.; Begmanova, M.; Tolebaeva, A.; Koishekenova, G.; Zaypanova, S.; Pilyugina, A.; et al. Ecological risk assessment and long-term environmental pollution caused by obsolete undisposed organochlorine pesticides. J. Environ. Sci. Health B 2021, 56, 490–502.
- Chitara, M.K.; Singh, R.P.; Gupta, P.K.; Mishra, D.; Jatav, S.S.; Sharma, S.; Jatav, H.S. The risk associated with crop ecosystem management and pesticides pollution. In Ecosystem Services: Types, Management and Benefits; Jatav, H.S., Rajput, V.D., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2022; pp. 151–163.
- Bhandari, G.; Atreya, K.; Scheepers, P.T.; Geissen, V. Concentration and distribution of pesticide residues in soil: Non-dietary human health risk assessment. Chemosphere 2020, 253, 126594.
- Singh, S.P.; Singh, M.K. Soil pollution and human health. In Plant Responses to Soil Pollution; Singh, P., Singh, S.K., Prasad, S.M., Eds.; Springer: Singapore, 2020; pp. 205–220.
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338.
- Narayanan, M.; Kandasamy, S.; He, Z.; Kumarasamy, S. Ecological impacts of pesticides on soil and water ecosystems and its natural degradation process. In Pesticides in the Natural Environment; Singh, P., Singh, S., Sillanpää, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–49.
- Sarker, S.; Akbor, M.A.; Nahar, A.; Hasan, M.; Islam, A.R.M.T.; Siddique, M.A.B. Level of pesticides contamination in the major river systems: A review on South Asian countries perspective. Heliyon 2021, 7, e07270.
- Tao, Y.; Liu, J.; Xu, Y.; Liu, H.; Yang, G.; He, Y.; Xu, J.; Lu, Z. Suspecting screening “known unknown” pesticides and transformation products in soil at pesticide manufacturing sites. Sci. Total Environ. 2022, 808, 152074.
- Bagheri, A.; Emami, N.; Damalas, C.A. Farmers’ behavior towards safe pesticide handling: An analysis with the theory of planned behavior. Sci. Total Environ. 2021, 751, 141709.
- Islam, M.A.; Amin, S.N.; Rahman, M.A.; Juraimi, A.S.; Uddin, M.K.; Brown, C.L.; Arshad, A. Chronic effects of organic pesticides on the aquatic environment and human health: A review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100740.
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarmi, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in drinking water—A review. Int. J. Environ. Res. Public Health 2021, 18, 468.
- Boudh, S.; Singh, J.S. Pesticide contamination: Environmental problems and remediation strategies. In Emerging and Eco-Friendly Approaches for Waste Management; Bharagava, R., Chowdhary, P., Eds.; Springer: Singapore, 2019; pp. 245–269.
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, distribution pathways and effects on human health–a review. Toxicol. Rep. 2021, 8, 1179–1192.
- Wang, X.; Sial, M.U.; Bashir, M.A.; Bilal, M.; Raza, Q.U.A.; Ali Raza, H.M.; Rehim, A.; Geng, Y. Pesticides xenobiotics in soil ecosystem and their remediation approaches. Sustainability 2022, 14, 3353.
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020, 60, 125–138.
- Raffa, C.M.; Chiampo, F. Bioremediation of agricultural soils polluted with pesticides: A review. Bioengineering 2021, 8, 92.
- Karimi, H.; Mahdavi, S.; Asgari Lajayer, B.; Moghiseh, E.; Rajput, V.D.; Minkina, T.; Astatkie, T. Insights on the bioremediation technologies for pesticide-contaminated soils. Environ. Geochem. Health 2022, 44, 1329–1354.
- Karas, P.; Metsoviti, A.; Zisis, V.; Ehaliotis, C.; Omirou, M.; Papadopoulou, E.S.; Menkissoglou-Spiroudi, U.; Manta, S.; Komiotis, D.; Karpouzas, D.G. Dissipation, metabolism and sorption of pesticides used in fruit-packaging plants: Towards an optimized depuration of their pesticide-contaminated agro-industrial effluents. Sci. Total Environ. 2015, 530, 129–139.
- Papazlatani, C.V.; Karas, P.A.; Tucat, G.; Karpouzas, D.G. Expanding the use of biobeds: Degradation and adsorption of pesticides contained in effluents from seed-coating, bulb disinfestation and fruit-packaging activities. J. Environ. Manag. 2019, 248, 109221.
- Rezende, S.; Cesio, M.V.; Archondo, L.; Russi, C.; Martínez, P.; Rivero, A.; Besil, N. Pilot study of biobeds application for the remediation of citrus agro-industrial wastewaters. Int. J. Environ. Anal. Chem. 2021, 1–17.
- Lescano, M.; Fussoni, N.; Vidal, E.; Zalazar, C. Biodegradation of pesticide-contaminated wastewaters from a formulation plant employing a pilot scale biobed. Sci. Total Environ. 2022, 807, 150758.
- Papazlatani, C.V.; Karas, P.A.; Lampronikou, E.; Karpouzas, D.G. Using biobeds for the treatment of fungicide-contaminated effluents from various agro-food processing industries: Microbiome responses and mobile genetic element dynamics. Sci. Total Environ. 2022, 823, 153744.
- Romero, E.; Delgado-Moreno, L.; Nogales, R. Pesticide dissipation and enzyme activities in ungrassed and grassed biomixtures, composed of winery wastes, used in biobed bioremediation systems. Water Air Soil Pollut. 2019, 230, 33.
- Carniel, L.S.C.; Niemeyer, J.C.; de Oliveira Filho, L.C.I.; Alexandre, D.; Gebler, L.; Klauberg-Filho, O. Are there any risks of the disposal of pesticide effluents in soils? Biobed system meets ecotoxicology ensuring safety to soil fauna. Ecotoxicology 2020, 29, 1409–1421.
- Pinto, A.P.; Lopes, M.E.; Dordio, A.; Castanheiro, J.E.F. Bioaugmentation an effective strategy to improve the performance of biobeds: A review. In Agrochemicals Detection, Treatment and Remediation; Prassad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–240.
- Córdova-Méndez, E.A.; Góngora-Echeverría, V.R.; González-Sánchez, A.; Quintal-Franco, C.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Pesticide treatment in biobed systems at microcosms level under critical moisture and temperature range using an Orthic Solonchaks soil from southeastern Mexico amended with corn husk as support. Sci. Total Environ. 2021, 772, 145038.
- Özkara, A.; Akyıl, D.; Konuk, M. Pesticides, environmental pollution, and health. In Environmental Health Risk-Hazardous Factors to Living Species; Larramendy, M.L., Soloneski, S., Eds.; Intech Open: London, UK, 2016; pp. 3–27.
- Tang, F.H.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210.
- Dhananjayan, V.; Jayakumar, S.; Ravichandran, B. Conventional methods of pesticide application in agricultural field and fate of the pesticides in the environment and human health. In Controlled Release of Pesticides for Sustainable Agriculture; Rakhimol, K.R., Sabu, T., Tatiana, V., Jayachandran, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–37.
- Le Cor, F.; Slaby, S.; Dufour, V.; Iuretig, A.; Feidt, C.; Dauchy, X.; Banas, D. Occurrence of pesticides and their transformation products in headwater streams: Contamination status and effect of ponds on contaminant concentrations. Sci. Total Environ. 2021, 788, 147715.
- Brhich, A.; Ait Sidi Brahim, M.; Merzouki, H.; Chatoui, R.; Merzouki, M. Fate and impact of pesticides: Environmental and human health issues. In Nutrition and Human Health; Chatoui, H., Merzouki, M., Moummou, H., Tilaoui, M., Saadaoui, N., Brhich, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 41–53.
- Dias, L.D.A.; Gebler, L.; Niemeyer, J.C.; Itako, A.T. Destination of pesticide residues on biobeds: State of the art and future perspectives in Latin America. Chemosphere 2020, 248, 126038.
- Delgado-Moreno, L.; Bazhari, S.; Nogales, R.; Romero, E. Innovative application of biobed bioremediation systems to remove emerging contaminants: Adsorption, degradation and bioaccesibility. Sci. Total Environ. 2019, 651, 990–997.
- Torstensson, L.; Castillo, M. Use of biobeds in Sweden to minimize environmental spillages from agricultural spraying equipment. Pestic. Outlook 1997, 8, 24–27.
- Torstensson, L. Experiences of biobeds in practical use in Sweden. Pestic. Outlook 2000, 11, 206–211.
- Castillo, M.D.P.; Torstensson, L.; Stenström, J. Biobeds for environmental protection from pesticide use—A review. J. Agric. Food Chem. 2008, 56, 6206–6219.
- Biobeds.org. The International Biobed Site. 2022. Available online: https://bricksite.com/biobed (accessed on 1 February 2023).
- Henriksen, V.V.; Helweg, A.; Spliid, N.H.; Felding, G.; Stenvang, L. Capacity of model biobeds to retain and degrade mecoprop and isoproturon. Pest Manag. Sci. 2003, 59, 1076–1082.
- Fogg, P.; Boxall, A.B.; Walker, A.; Jukes, A. Leaching of pesticides from biobeds: Effect of biobed depth and water loading. J. Agric. Food Chem. 2004, 52, 6217–6227.
- Castillo, M.D.P.; Torstensson, L. Biobeds-Biotechnology for environmental protection from pesticide pollution. In Methods and Techniques for Cleaning-Up Contaminated Sites; Annable, M.D., Teodorescu, M., Hlavinek, P., Diels, L., Eds.; Springer: Cham, Switzerland, 2008; pp. 145–151.
- Cooper, R.J.; Fitt, P.; Hiscock, K.M.; Lovett, A.A.; Gumm, L.; Dugdale, S.J.; Rambohul, J.; Williamson, A.; Noble, L.; Beamish, J.; et al. Assessing the effectiveness of a three-stage on-farm biobed in treating pesticide contaminated wastewater. J. Environ. Manag. 2016, 181, 874–882.
- Saez, J.M.; Bigliardo, A.L.; Raimondo, E.E.; Briceño, G.E.; Polti, M.A.; Benimeli, C.S. Lindane dissipation in a biomixture: Effect of soil properties and bioaugmentation. Ecotoxicol. Environ. Saf. 2018, 156, 97–105.
- Castillo, M.D.P.; Torstensson, L. Effect of biobed composition, moisture, and temperature on the degradation of pesticides. J. Agric. Food Chem. 2007, 55, 5725–5733.
- De Wilde, T.; Spanoghe, P.; Debaer, C.; Ryckeboer, J.; Springael, D.; Jaeken, P. Overview of on-farm bioremediation systems to reduce the occurrence of point source contamination. Pest Manag. Sci. 2007, 63, 111–128.
- Karanasios, E.; Karpouzas, D.G.; Tsiropoulos, N.G. Key parameters and practices controlling pesticide degradation efficiency of biobed substrates. J. Environ. Sci. Health B 2012, 47, 589–598.
- Urrutia, C.; Rubilar, O.; Tortella, G.R.; Diez, M.C. Degradation of pesticide mixture on modified matrix of a biopurification system with alternatives lignocellulosic wastes. Chemosphere 2013, 92, 1361–1366.
- Karanasios, E.; Tsiropoulos, N.G.; Karpouzas, D.G.; Menkissoglu-Spiroudi, U. Novel biomixtures based on local Mediterranean lignocellulosic materials: Evaluation for use in biobed systems. Chemosphere 2010, 80, 914–921.
- Diez, M.C.; Tortella, G.R.; Briceño, G.; Castillo, M.D.P.; Díaz, J.; Palma, G.; Altamirano, C.; Calderón, C.; Rubilar, O. Influence of novel lignocellulosic residues in a biobed biopurification system on the degradation of pesticides applied in repeatedly high doses. Electron. J. Biotechnol. 2013, 16, 1–11.
- Góngora-Echeverría, V.R.; Martin-Laurent, F.; Quintal-Franco, C.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Agricultural effluent treatment in biobed systems using novel substrates from southeastern Mexico: The relationship with physicochemical parameters of biomixtures. Environ. Sci. Pollut. Res. 2017, 24, 9741–9753.
- Gao, W.; Liang, J.; Pizzul, L.; Feng, X.M.; Zhang, K.; del Pilar Castillo, M. Evaluation of spent mushroom substrate as substitute of peat in chinese biobeds. Int. Biodeterior. Biodegrad. 2015, 98, 107–112.
- Yarpuz-Bozdogan, N.; Bozdogan, A.M.; Daglioglu, N.; Sagliker, H. Determination of the effect of agricultural wastes alternative to peat in biobed. Fresenius Environ. Bull. 2020, 29, 9906–9913.
- Masís-Mora, M.; Lizano-Fallas, V.; Tortella, G.; Beita-Sandí, W.; Rodríguez-Rodríguez, C.E. Removal of triazines, triazoles and organophophates in biomixtures and application of a biopurification system for the treatment of laboratory wastewaters. Chemosphere 2019, 233, 733–743.
- Acosta-Sánchez, A.; Soto-Garita, C.; Masís-Mora, M.; Cambronero-Heinrichs, J.C.; Rodríguez-Rodríguez, C.E. Impaired pesticide removal and detoxification by biomixtures during the simulated pesticide application cycle of a tropical agricultural system. Ecotoxicol. Environ. Saf. 2020, 195, 110460.
- Vischetti, C.; Monaci, E.; Casucci, C.; De Bernardi, A.; Cardinali, A. Adsorption and degradation of three pesticides in a vineyard soil and in an organic biomix. Environments 2020, 7, 113.
- Kumari, A.; Kumari, U.; Gupta, S.; Singh, N. Azoxystrobin and imidacloprid degradation in biobed setup under laboratory conditions. J. Environ. Anal. Chem. 2021, 103, 2292–2299.
- Kumari, U.; Banerjee, T.; Narayanan, N.; Yadav, S.; Singh, N. Ash and biochar mixed biomixtures to degrade co-applied atrazine and fipronil in bio-augmented biobeds. J. Environ. Anal. Chem. 2022.
- Delgado-Moreno, L.; Nogales, R.; Romero, E. Biodegradation of high doses of commercial pesticide products in pilot-scale biobeds using olive-oil agroindustry wastes. J. Environ. Manag. 2017, 204, 160–169.
- Delgado-Moreno, L.; Nogales, R.; Romero, E. Wastes from the olive oil production in sustainable bioremediation systems to prevent pesticides water contamination. Int. J. Environ. Sci. Technol. 2017, 14, 2471–2484.
- Dias, L.D.A.; Itako, A.T.; Gebler, L.; Tolentino Júnior, J.B.; Pizzutti, I.R.; Fontana, M.E.; Janish, B.D.; Niemeyer, J.C. Pine litter and vermicompost as alternative substrates for biobeds: Efficiency in pesticide degradation. Water Air Soil Pollut. 2021, 232, 283.
- Fenoll, J.; Garrido, I.; Hellín, P.; Flores, P.; Vela, N.; Navarro, S. Use of different organic wastes as strategy to mitigate the leaching potential of phenylurea herbicides through the soil. Environ. Sci. Pollut. Res. 2015, 22, 4336–4349.
- Kravvariti, K.; Tsiropoulos, N.G.; Karpouzas, D.G. Degradation and adsorption of terbuthylazine and chlorpyrifos in biobed biomixtures from composted cotton crop residues. Pest Manag. Sci. 2010, 66, 1122–1128.
- Lemerhyeratte, A.; Zougagh, M.; El Mouden, O.I.; Salghi, R.; Bazzi, L.; Hormatallah, A.; Zine, S. Biobed system to reduce four pesticide organophosphorus point contamination at farm level. Orient. J. Chem. 2010, 26, 15–22.
- Ruiz-Hidalgo, K.; Chin-Pampillo, J.S.; Masís-Mora, M.; Carazo-Rojas, E.; Rodríguez-Rodríguez, C.E. Optimization of a fungally bioaugmented biomixture for carbofuran removal in on-farm biopurification systems. Water Air Soil Pollut. 2016, 227, 3.
- Castro-Gutiérrez, V.; Masís-Mora, M.; Carazo-Rojas, E.; Mora-López, M.; Rodríguez-Rodríguez, C.E. Impact of oxytetracycline and bacterial bioaugmentation on the efficiency and microbial community structure of a pesticide-degrading biomixture. Environ. Sci. Pollut. Res. 2018, 25, 11787–11799.
- Cessna, A.J.; Knight, J.D.; Ngombe, D.; Wolf, T.M. Effect of temperature on the dissipation of seven herbicides in a biobed matrix. Can. J. Soil Sci. 2017, 97, 717–731.
- El Bakouri, H.; Morillo, J.; Usero, J.; Vanderlinden, E.; Vidal, H. Effectiveness of acid-treated agricultural stones used in biopurification systems to avoid pesticide contamination of water resources caused by direct losses: Part I. Equilibrium experiments and kinetics. Bioresour. Technol. 2010, 101, 5084–5091.
- Karanasios, E.; Tsiropoulos, N.G.; Karpouzas, D.G.; Ehaliotis, C. Degradation and adsorption of pesticides in compost-based biomixtures as potential substrates for biobeds in Southern Europe. J. Agric. Food Chem. 2010, 58, 9147–9156.
- Rojas, R.; Morillo, J.; Usero, J.; Vanderlinden, E.; El Bakouri, H. Adsorption study of low-cost and locally available organic substances and a soil to remove pesticides from aqueous solutions. J. Hydrol. 2015, 520, 461–472.
- Briceño, G.; Tortella, G.; Rubilar, O.; Palma, G.; Diez, M.C. Advances in Chile for the treatment of pesticide residues: Biobeds technology. In Bioremediation in Latin America: Current Research and Perspectives; Alvarez, A., Polti, M., Eds.; Springer: Cham, Switzerland, 2014; pp. 53–68.
- Kumari, U.; Banerjee, T.; Singh, N. Evaluating ash and biochar mixed biomixtures for atrazine and fipronil degradation. Environ. Technol. Innov. 2021, 23, 101745.
- Karas, P.A.; Makri, S.; Papadopoulou, E.S.; Ehaliotis, C.; Menkissoglu-Spiroudi, U.; Karpouzas, D.G. The potential of organic substrates based on mushroom substrate and straw to dissipate fungicides contained in effluents from the fruit-packaging industry–Is there a role for Pleurotus ostreatus? Ecotoxicol. Environ. Saf. 2016, 124, 447–454.
- Adak, T.; Mahapatra, B.; Swain, H.; Patil, N.B.; Gowda, G.B.; Annamalai, M.; Pokhare, S.S.; Meena, K.S.; Rath, P.C.; Jena, M. Indigenous biobed to limit point source pollution of imidacloprid in tropical countries. J. Environ. Manag. 2020, 272, 111084.
- Mohapatra, D.; Rath, S.K.; Mohapatra, P.K. Bioremediation of insecticides by white-rot fungi and its environmental relevance. In Mycoremediation and Environmental Sustainability; Prasad, R., Ed.; Springer: Cham, Switzerland, 2018; pp. 181–212.
- Chandra, P.; Enespa. Fungal Enzymes for Bioremediation of Contaminated Soil. In Recent Advancement in White Biotechnology through Fungi; Yadav, A., Singh, S., Mishra, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 189–215.
- Singh, R.K.; Tripathi, R.; Ranjan, A.; Srivastava, A.K. Fungi as potential candidates for bioremediation. In Abatement of Environmental Pollutants; Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–191.
- Kathiravan, A.; Gnanadoss, J.J. White-rot fungi-mediated bioremediation as a sustainable method for xenobiotic degradation. Environ. Exp. Biol. 2021, 19, 103–119.
- Tomer, A.; Singh, R.; Singh, S.K.; Dwivedi, S.A.; Reddy, C.U.; Keloth, M.R.A.; Rachel, R. Role of fungi in bioremediation and environmental sustainability. In Mycoremediation and Environmental Sustainability; Prasad, R., Nayak, S.C., Kharwar, R.N., Dubey, N.K., Eds.; Springer: Cham, Switzerland, 2021; pp. 187–200.
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905.
- Singh, N.; Kumar, A.; Sharma, B. Role of fungal enzymes for bioremediation of hazardous chemicals. In Recent Advancement in White Biotechnology through Fungi; Yadav, A., Singh, S., Mishra, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 237–256.
- Zhuo, R.; Fan, F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 146132.
- Lin, S.; Wei, J.; Yang, B.; Zhang, M.; Zhuo, R. Bioremediation of organic pollutants by white rot fungal cytochrome P450: The role and mechanism of CYP450 in biodegradation. Chemosphere 2022, 301, 134776.
- Ortiz-Hernández, M.L.; Sánchez-Salinas, E.; Dantán-González, E.; Castrejón-Godínez, M.L. Pesticide biodegradation: Mechanisms, genetics and strategies to enhance the process. In Biodegradation-Life of Science; Chamy, R., Rosenkranz, F., Eds.; IntechOpen: London, UK, 2013; pp. 251–287.
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; López-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial degradation of organophosphate pesticides: A review. Pedosphere 2018, 28, 190–208.
- Kumar, S.S.; Ghosh, P.; Malyan, S.K.; Sharma, J.; Kumar, V. A comprehensive review on enzymatic degradation of the organophosphate pesticide malathion in the environment. J. Environ. Sci. Health C 2019, 37, 288–329.
- Dave, S.; Das, J. Role of microbial enzymes for biodegradation and bioremediation of environmental pollutants: Challenges and future prospects. In Bioremediation for Environmental Sustainability; Saxena, G., Kumar, V., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 325–346.
- Saravanan, A.; Kumar, P.S.; Vo, D.V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. A review on catalytic-enzyme degradation of toxic environmental pollutants: Microbial enzymes. J. Hazard. Mater. 2021, 419, 126451.
- Bergsveinson, J.; Perry, B.J.; Sheedy, C.; Braul, L.; Reedyk, S.; Gossen, B.D.; Yost, C.K. Identifying the core bacterial and fungal communities within four agricultural biobeds used for the treatment of pesticide rinsates. J. Appl. Microbiol. 2018, 125, 1333–1342.
- Góngora-Echeverría, V.R.; Quintal-Franco, C.; Arena-Ortiz, M.L.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Identification of microbial species present in a pesticide dissipation process in biobed systems using typical substrates from southeastern Mexico as a biomixture at a laboratory scale. Sci. Total Environ. 2018, 628, 528–538.
- Russell, J.N.; Perry, B.J.; Bergsveinson, J.; Freeman, C.N.; Sheedy, C.; Nilsson, D.; Braul, L.; Yost, C.K. Metagenomic and metatranscriptomic analysis reveals enrichment for xenobiotic-degrading bacterial specialists and xenobiotic-degrading genes in a Canadian prairie two-cell biobed system. Environ. Microbiol. Rep. 2021, 13, 720–727.
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71.
- Magan, N.; Gouma, S.; Fragoeiro, S.; Shuaib, M.E.; Bastos, A.C. Bacterial and fungal bioremediation strategies. In Microbial Biodegradation and Bioremediation; Das, S., Dash, H.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 193–212.
- Fernández-Alberti, S.; Rubilar, O.; Tortella, G.R.; Diez, M.C. Chlorpyrifos degradation in a biomix: Effect of pre-incubation and water holding capacity. J. Soil Sci. Plant Nutr. 2012, 12, 785–799.
- Tortella, G.R.; Rubilar, O.; Castillo, M.; Cea, M.; Mella-Herrera, R.; Diez, M.C. Chlorpyrifos degradation in a biomixture of biobed at different maturity stages. Chemosphere 2012, 88, 224–228.
- Kumari, A.; Singh, N.; Ramakrishnan, B. Parameters affecting azoxystrobin and imidacloprid degradation in biobed substrates in the North Indian tropical environment. J. Environ. Sci. Health B 2019, 54, 843–857.
- Basford, B. BIOBEDS. Outlooks Pest Manag. 2010, 21, 78–80.
- Spliid, N.H.; Helweg, A.; Heinrichson, K. Leaching and degradation of 21 pesticides in a full-scale model biobed. Chemosphere 2006, 65, 2223–2232.
