钒氧化还原液流电池(Vanadium redox flow batteries (VRFBs) are promising candidates for large-scale energy storage, and the electrolyte plays a critical role in chemical–electrical energy conversion. However, the operating temperature of VRFBs is limited to 10–40 °C because of the stability of the electrolyte. To overcome this, various chemical species are added, but the progress and mechanism have not been summarized and discussed yet. RFB)是大规模储能的有希望的候选者,电解质在化学-电能转换中起着关键作用。然而,由于电解质的稳定性,VRFB的工作温度被限制在10-40°C。为了克服这一点,添加了各种化学物质。
- vanadium redox flow batteries
- electrolyte additives
- electrochemical performance
- complexation
- electrostatic repulsion
- growth inhibition
- modifying electrode
1. Introduction简介
VRFBs, first proposed by Skyllas-Kazacos in 1986 [19], consist of three key factors: electrodes, an ion exchange membrane, and electrolytes [20–23]. Among them, the electrolyte, as the core part of the vanadium battery system, greatly affects the energy density and overall performance of the battery. It can generally be divided into positive and negative electrolytes, which correspond to the sulfuric acid solutions of the V(IV)/V(V) and V(II)/V(III) redox couples in Equations (1)–(3) [24], respectively. The same elements at different oxidation states can be converted to one another at the electrodes, achieving the chemical–electrical energy conversion, as shown in Figure 1.
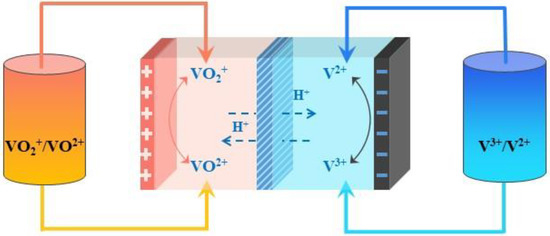
Figure 图1. Vanadium redox flow battery schematic.
The vanadium electrolyte is generally prepared through the methods of physical dissolution, chemical reduction, electrolysis, and chemistry–electrolysis coupling [25] Among them, the chemistry–electrolysis coupling is the dominant method, which takes high-purity V
钒液流电池原理图。2
5 as the raw material and adds reducing agents such as H
2
2
4 [26], SO
2, [27], and elemental sulfur to the sulfuric acid to prepare the V(IV) electrolyte, and then reduces it by electrolysis to obtain the V(III) electrolyte. If the operating temperature of the vanadium electrolyte is higher than 40 °C or lower than 10 °C, both the electrolyte stability and energy density of vanadium batteries will decrease, accompanied by capacity loss and battery failure [28]. To solve this problem, additives are added to the electrolyte [29] to improve its stability and optimize the electrochemical kinetics, so as to expand the operating temperature range and raise the energy density of the VRFB. For example, the introduction of ammonium dihydrogen phosphate [30] and acidic amino acid [31] as additives can enhance the high-temperature stability of electrolytes, and ammonium and α-lactose monohydrate [32] have been used to improve the low-temperature stability of electrolytes. Additionally, additives such as polyacrylic acid (PAA) [33] can strengthen electrochemical mass transfer.
Additives in vanadium electrolytes, generally classified as inorganics, organics, and compounds, exhibit different microscopic mechanisms, including complexation, electrostatic repulsion, and growth inhibition. Specifically, while complexation improves anti-precipitation properties by changing the distribution of electron clouds, electrostatic repulsion reduces the agglomeration of V(V) ions by enhancing the dispersion effect of V(V) ions from each other. Growth inhibition, on the other hand, lowers the size of settled particles by impeding the growth kinetics of V
2
5. Furthermore, the electrochemical mass transfer enhancers in vanadium electrolytes mainly perform hydrophilic modification to enhance electrochemical kinetics, such as MSA, which can be adsorbed on the electrodes to increase the active sites. This adsorption behavior can promote redox reaction kinetics in the interface and optimize the electrochemical performance of the VRFB. Therefore, the introduction of additives can effectively increase the operating temperature range of the vanadium electrolyte, providing effective technical support for the large-scale application of the VRFB.
2. The Function Mechanisms of Additives
The function mechanisms of additives involved in electrolyte performance improvement are still under investigation. Because additives are mainly classified into stabilizing agents, including complexing agents and electrostatic repulsion agents, and growth inhibitors and electrochemical enhancers, the stabilizing mechanisms and enhancement mechanisms will be discussed separately. Whereas the principal stabilizing mechanisms involve complexation, electrostatic repulsion, and growth inhibition, the enhancement mechanism of electrochemical mass transfer mainly involves additives’ hydrophilic modification of the electrode.
二、添加剂的作用机理
添加剂参与电解质性能改善的功能机制仍在研究中。由于添加剂主要分为稳定剂,包括络合剂和静电排斥剂,以及生长抑制剂和电化学增强剂,因此稳定机理和增强机理将单独讨论。电化学传质的主要稳定机理涉及络合、静电斥力和生长抑制,电化学传质的增强机理主要涉及添加剂对电极的亲水改性。2.1. 稳定机制
3.1.1. Complexation
2.1.1. 络合
Complexation is the foremost stabilizing mechanism of additives, and is applied to both inorganic and organic additives. As illustrated in Figure 2, ions (Cl
络合是添加剂最重要的稳定机理,适用于无机和有机添加剂。如图2所示,离子(Cl−, H
, H2PO
采购订单4−) or functional groups (-COOH, -NH
)或官能团(-COOH,-NH2, -OH, -SO
, -哦, -所以3H) carrying lone-pair electrons are capable of coordinating with hydrated vanadium ions in the electrolyte to form a more stable intermediate with V–O–S, V–O–P, V–O–Cl, V–O–N, and so on, thereby effectively reducing the formation of V–O–V and inhibiting V
H)携带孤对电子能够与电解质中的水合钒离子配位,与V-O-S、V-O-P、V-O-Cl、V-O-N等形成更稳定的中间体,从而有效减少V-O-V的形成,抑制V2
O5 precipitation. Subsequently, the electron density of vanadium will increase and the local positive charge of vanadium will decrease [50]. Furthermore, the reaction barrier of forming V–O–X (X is the core element of additives) is generally lower than that of forming V–O–V from V
降水。随后,钒的电子密度会增加,钒的局部正电荷会降低[48]。此外,形成V-O-X(X是添加剂的核心元素)的反应势垒通常低于从V形成V-O-V的反应势垒2
O5, which considerably reduces the generation selectivity of V
,这大大降低了V的生成选择性2
O5 precipitation [51,52].
降水[49,50]。
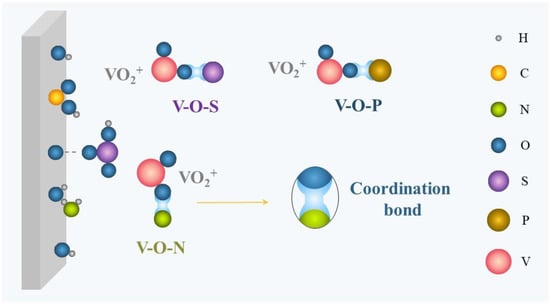
Figure 图2. Schematic of complexation.
In addition, a large number of studies have shown that complexation behaviors can be impacted by the geometries of hydrated vanadium ions in different valences, the synergistic effect of additives, and other supplementary factors. For one, the geometries of hydrated ions of vanadium in diverse valences are different and the complexing capacity is positively correlated with the stability of the additives to vanadium ions in all valences. Clarifying the complexation of vanadium ions with additives in each valence is necessary for both stabilization maximization and additive selection. For another, adopting synergistic effects can intensify the stabilization effect and maintain a balance between various ions, such as phosphate and ammonium. Finally, other supplementary factors can be involved, including introducing double additives to form a competing relationship, modifying the electrodes of VRFBs, integrating a thermally regenerative electrochemical cycle (TREC) into the VRFBs, and so on. Researchers should be aware of the above-mentioned considerations when selecting additives.
Taking H
络合示意图。3PO
4 as an example, the transformation path of V(V) in phosphates-added electrolytes is: [VO
2(H
2
2
+ → [VO(OH)
2(H
2O)]
+ → VO(OH)3 [53]. The VO(OH)
3 intermediate can form a compound containing a V–O–P bond with H
3PO
4. Moreover, the activation energy of this reaction is generally lower than that of the formation of the V–O–V bond, which could effectively avoid V
2
5 precipitation, as presented in Figure 3. Furthermore, the dominant form of the anions is H
2PO
4− after phosphate additives are added to the positive electrolyte [51]. In this case, due to the partial dimerization of V(V), the sulfate is coordinated to two oxygen atoms in a bridging or bidentate coordination. This can be explained by the coordination of H
3PO
4 or by the rearrangement of the complexation pattern due to dimerization.
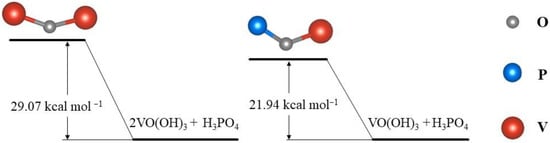
2.1.2. Electrostatic Repulsion
In electrostatic repulsion, a common stabilizing mechanism, ions or functional groups of additives, can be adsorbed on vanadium ions by electrostatic attraction, mainly including -COOH, -OH, -SO3H, -S-, -NH2, and so on. This adsorption behavior promotes the formation of ionic agglomerates with vanadium ions as the core element, which can enhance the outer layer charge and generate electrostatic repulsion to varying degrees, as shown in Figure 5. Meanwhile, due to the large core–shell spatial structure, the steric hindrance of this agglomerate encapsulating vanadium ions can strengthen this repulsion effect, making vanadium ions more dispersed, to inhibit precipitation [52]. A proliferation of studies has demonstrated the dominance of electrostatic repulsion in the stabilizing mechanism of organic additives, but the application of this principle in inorganic additives is minimal. More specifically, anionic functional groups produce negatively charged groups (e.g., -COO−) in the electrolyte, which brings about electrostatic attraction with the positively charged vanadium ions to form agglomerates. Polar groups (e.g., -NH2) tend to be adsorbed on vanadium ions based on the like-dissolves-like theory to generate external charge layers, boosting the repulsion effect [32,33,53].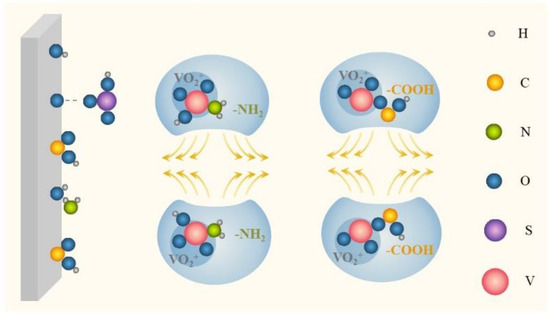
2.1.3. Growth Inhibition
Growth inhibition, an uncommon stabilizing mechanism, means that some additives can inhibit the growth kinetics of V2O5 precipitates in the vanadium electrolyte. As shown in Figure 6, the V2O5 precipitates can generally grow with increasing time without additives. However, after introducing some specific additives, the surfaces of the nucleation sites of the V2O5 were adsorbed by various molecules to lower the growth rate of V2O5 precipitates, lengthening the induction time of precipitation and reducing the sizes of V2O5 particles [43,52,54].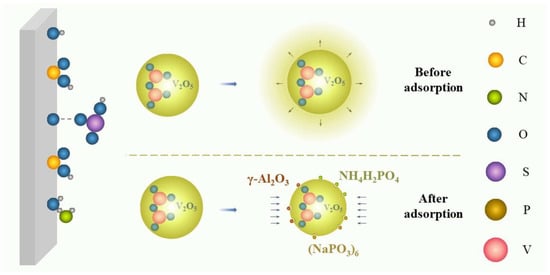
Figure 图6. Schematic of growth inhibition.
2.2. Electrochemical Mass Transfer Enhancement Mechanism
As Figure 7 shows, the Helmholtz model depicts how the opposite charges can form a set of polar plates in an electrolyte due to mutual attraction in the electrode–electrolyte interface, further forming a capacitor to store energy, which is called the electric double layer. The whole process of redox reaction is divided into a reaction region and a transfer region, whose rates are co-controlled by electron transfer and migrating mass transfer. The high charge gradient in the region of the electric double layer reduces the mass transfer rate of the solid–liquid interface and accelerates the reaction rate. Therefore, in the transfer region, the rate of transfer usually slows down, thus limiting the electrochemical performance, including energy efficiency, capacity retention rate, and the properties of charging and discharging. Accordingly, the mass transfer of electric double layers becomes an important speed-controlled step of electrochemical kinetics.
生长抑制示意图。2.2. 电化学传质增强机理
如图7所示,亥姆霍兹模型描述了由于电极-电解质界面中的相互吸引,相反的电荷如何在电解质中形成一组极板,进一步形成电容器来存储能量,称为双电层。氧化还原反应的整个过程分为反应区和转移区,其速率由电子转移和迁移传质共同控制。双电层区域的高电荷梯度降低了固液界面的传质速率,加快了反应速率。因此,在转移区,转移速率通常会减慢,从而限制了电化学性能,包括能效、容量保持率和充放电性能。因此,双电层的传质成为电化学动力学的重要调速步骤。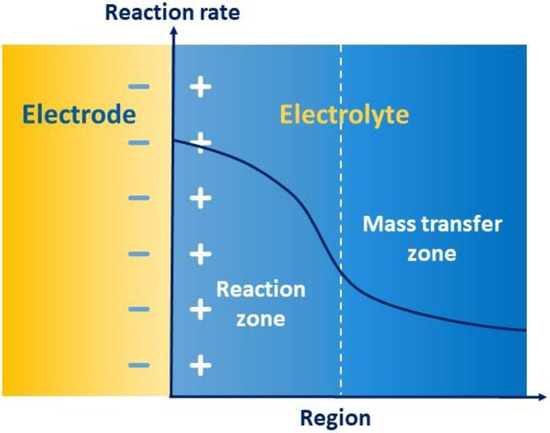
Figure 图7. Schematic of the mass transfer of electric double layers.
The foremost mechanism of enhancing electrochemical mass transfer is that additives can be adsorbed on the surface of the electrode to promote active sites to form a “hydrophilic modification” to electrodes, mainly including hydroxyl, the sulfonic group, pyridyl, and other hydrophilic functional groups, or certain ions [32,57], as illustrated in Figure 8. This modifying behavior to electrodes can activate the interfacial activity between electrodes and the electrolyte, accelerating both the redox reaction of vanadium ions in all valences and the migration mass transfer. Alternatively, additives, such as taurine, MSA, PPS, benzoyl peroxide, and so on, can reduce the overpotential of the VRFB to facilitate the migrating mass transfer, reducing the resistance of the electric double layer and enhancing the kinetics of redox reactions [58–62].
双电层传质示意图。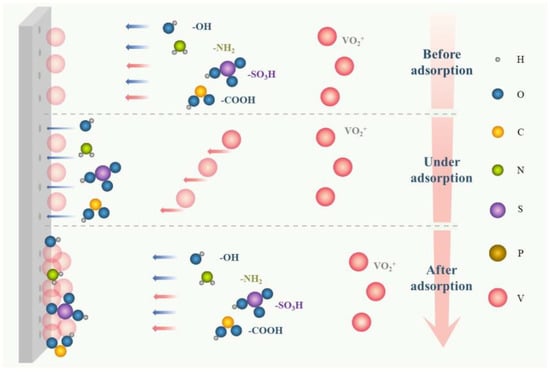
Figure 图8. Schematic of the mechanism of electrochemical mass transfer enhancers.
电化学传质增强剂机理示意图.