Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Wei Qiao.
Anaerobic digestion is one of the most widely used treatment methods for animal manure. Chicken manure has high methane production potential and is thus a suitable substrate for biogas plants.
- anaerobic digestion
- chicken manure
- ammonia inhibition
1. Introduction
Anaerobic digestion technology, the primary treatment strategy for animal manure, can efficiently treat organic matter to generate clean energy. In China, the annual output of animal manure reaches 3800 million tons [1]. This potential bioenergy from anaerobic treatment represents an ideal alternative to fossil energy sources and can reduce greenhouse gas emissions. Chicken manure is a bioenergy source that is very special as compared to pig and cow manure. Firstly, the total solids (TS) content of chicken manure is greater than 20%. It can be treated by high solids anaerobic digestion technology. However, a large amount of water is required to dilute chicken manure to about TS 10% before anaerobic treatment in the actual project [2]. This increases the production of digestate and operating costs. In addition, chicken manure contains considerable protein, uric acid, and other nitrogen-containing organic matter, with the nitrogen content usually being higher than 4% [3]. Anaerobic digestion technology, however, recommends a suitable carbon/nitrogen (C/N) ratio of 20–30 [4], while the C/N ratio of chicken manure is as low as 5–10 [5].
High ammonia level (total ammonia nitrogen, TAN > 3 g/L) is the primary bottleneck and is one of the main factors affecting microbial community structure and the methanogenic pathway in the anaerobic digestion of chicken manure. Previous studies demonstrated that hydrogenotrophic methanogens (converting H2 and CO2 into methane) were more tolerant of ammonia nitrogen than acetoclastic methanogens (cracking acetate to produce methane), which were dominant in high ammonia conditions [6]. However, recent studies found that acetate generated methane through syntrophic acetate oxidation combined with the hydrogenotrophic methanogenesis pathway under high ammonia stress [6]. Syntrophic oxidation is the primary metabolism of acetate at high ammonia levels [6]. Therefore, comprehensive knowledge of the links among ammonia levels, microbial community structure, and methanogenic pathways is critical to improving biogas production performance through improved operating strategies.
2. Ammonia Inhibition
2.1. Anaerobic Decomposition of Protein and Uric Acid in Chicken Manure
During the anaerobic treatment of chicken manure, process instability may occur due to high levels of ammonia nitrogen produced as a byproduct of protein and uric acid degradation. About 40%–70% of organic nitrogen in chicken manure comes from uric acid and 30%–60% from protein [10][7]. Protein was converted into amino acids by hydrolytic bacteria and then further converted into organic acids and ammonia by the action of acidogenic bacteria (Table 1). The protein degradation efficiency in chicken manure is less than 50% [11][8]. Notably, the degradation of proteins is complex and more sensitive to ammonia inhibition in the hydrolysis stage. For example, the peptone degradation efficiency was 50% under TAN levels of 2.0 g/L and rapidly decreased to 30% when the TAN increased to 5.0 g/L. However, peptone degradation almost ceased at a TAN of 6.5 g/L, and high ammonia levels mainly inhibit the deamination of peptone [12][9]. Therefore, due to the low degradability of proteins, uric acid degradation is one of the leading causes of high ammonia levels in the anaerobic digestion of chicken manure.Table 1.
Anaerobic degradation of protein and uric acid.
| The Methanation of Substrate | Equations |
|---|---|
| Protein | |
| C16H24O5N4 + 14.5 H2O = 8.25 CH4 + 3.75 CO2 + 4 NH4HCO3 | (1) |
| Uric acid by Clostridium purinolyticum: | |
| C5H4N4O3 = 0.58 CH3COOH + 0.25 HCOOH + 0.06 C2H5NO2 + 3.47 CO2 | (2) |
| Uric acid by Streptococcus sp.: | |
| C5H4N4O3 + HCOOH = CH3COOH + 4 CO2 + 4 NH3 | (3) |
| Uric acid by Bacteroides ternitidis: | |
| C5H4N4O3 = 0.75 CH3COOH + 3.5 CO2 + 4 NH3 | (4) |
2.2. Ammonia Inhibition Threshold for Anaerobic Consortia
Anaerobic digestion is performed by various microorganisms, with the degradation of substrate divided into hydrolysis, acidogenesis, acetogenesis, and methanogenesis (Figure 1) [17][14]. These steps need to be performed in balance in order to obtain stable operation. Ammonia nitrogen exists mainly as ammonium bicarbonate (NH4HCO3) under anaerobic alkaline conditions [18][15]. Ammonium bicarbonate can provide alkalinity, buffer the pH value of the digestate and enhance the buffering capacity of the system (Figure 1). In addition, minor TAN levels (0.05–0.2 g/L) benefit microorganisms [19][16]. High ammonia nitrogen levels (TAN > 3 g/L), however, can inhibit the activity of anaerobic consortia, especially acetoclastic methanogens. The anaerobic digestion process produces undesirably higher TAN concentrations, even more than 10 g/L for chicken manure [20][17]. TAN is composed of free ammonia nitrogen (FAN, NH3) and ammonium ions (NH4+). Temperature and pH modulate the balance between NH4+ and NH3, and the latter has been reported to be the leading cause of microbial inhibition [19][16].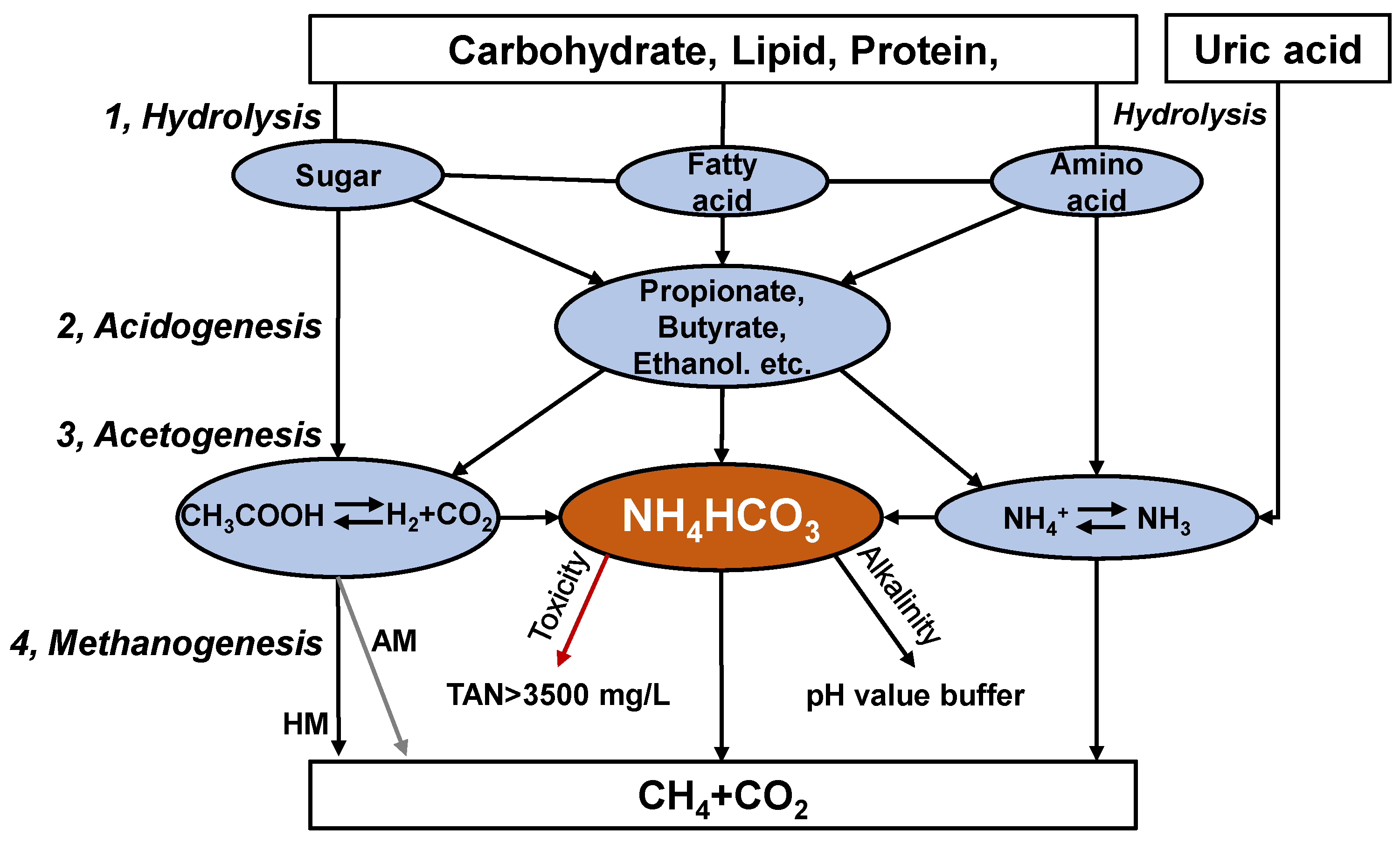
Figure 1.
Biological metabolism of organic matter in chicken manure during anaerobic digestion.
References
- Kang, Y.; Yang, Q.; Bartocci, P.; Wei, H.; Liu, S.S.; Wu, Z.; Zhou, H.; Yang, H.; Fantozzi, F.; Chen, H. Bioenergy in China: Evaluation of domestic biomass resources and the associated greenhouse gas mitigation potentials. Renew. Sustain. Energy Rev. 2020, 127, 109842.
- Li, K.; Liu, R.; Sun, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140.
- Ileleji, K.E.; Martin, C.; Jones, D. Chapter 17: Basics of Energy Production through Anaerobic Digestion of Livestock Manure. In Bioenergy; Academic Press: Cambridge, MA, USA, 2015; pp. 287–295.
- Wang, X.; Lu, X.; Li, F.; Yang, G. Effects of temperature and carbon-nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: Focusing on ammonia inhibition. PLoS ONE 2014, 9, e97265.
- Shapovalov, Y.; Zhadan, S.; Bochmann, G.; Salyuk, A.; Nykyforov, V. Dry Anaerobic Digestion of Chicken Manure: A Review. Appl. Sci. 2020, 10, 7825.
- Westerholm, M.; Moestedt, J.; Schnurer, A. Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl. Energy 2016, 179, 124–135.
- Fuchs, W.; Wang, X.; Gabauer, W.; Ortner, M.; Li, Z. Tackling ammonia inhibition for efficient biogas production from chicken manure: Status and technical trends in Europe and China. Renew. Sustain. Energy Rev. 2018, 97, 186–199.
- Niu, Q.; Takemura, Y.; Kubota, K.; Li, Y.Y. Comparing mesophilic and thermophilic anaerobic digestion of chicken manure: Microbial community dynamics and process resilience. Waste Manag. 2015, 43, 114–122.
- Gallert, C.; Bauer, S.; Winter, J. Effect of ammonia on the anaerobic degradation of protein by a mesophilic and thermophilic biowaste population. Appl. Microbiol. Biotechnol. 1998, 50, 495–501.
- Thong-On, A.; Suzuki, K.; Noda, S.; Inoue, J.; Kajiwara, S.; Ohkuma, M. Isolation and characterization of anaerobic bacteria for symbiotic recycling of uric acid nitrogen in the gut of various termites. Microbes Environ. 2012, 27, 186–192.
- Schnurer, A.; Schink, B.; Svensson, B.H. Clostridium ultunense sp. nov., a Mesophilic Bacterium Oxidizing Acetate in Syntrophic Association with a Hydrogenotrophic Methanogenic Bacterium. Int. J. Syst. Bacteriol. 1996, 46, 1145–1152.
- Dfirre, P.; Andreesen, J.R. Anaerobic Degradation of Uric Acid via Pyrimidine Derivatives. Arch. Microbiol. 1982, 131, 255–260.
- Potrikus, C.J.; Breznak, J.A. Anaerobic Degradation of Uric Acid by Gut Bacteria of termetest. Appl. Environ. Microbiol. 1980, 40, 125–132.
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557.
- Hao, T.; Xiao, Y.; Varjani, S. Transiting from the inhibited steady-state to the steady-state through the ammonium bicarbonate mediation in the anaerobic digestion of low-C/N-ratio food wastes. Bioresour. Technol. 2022, 351, 127046.
- Orhan, Y.; Burak, D. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911.
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899.
- Niu, Q.; Hojo, T.; Qiao, W.; Qiang, H.; Li, Y.-Y. Characterization of methanogenesis, acidogenesis and hydrolysis in thermophilic methane fermentation of chicken manure. Chem. Eng. J. 2014, 244, 587–596.
- Bi, S.; Qiao, W.; Xiong, L.; Ricci, M.; Adani, F.; Dong, R. Effects of organic loading rate on anaerobic digestion of chicken manure under mesophilic and thermophilic conditions. Renew. Energy 2019, 139, 242–250.
- Yin, D.; Qiao, W.; Negri, C.; Adani, F.; Fan, R.; Dong, R.-J. Enhancing hyper-thermophilic hydrolysis pretreatment of chicken manure for biogas production by in-situ gas phase ammonia stripping. Bioresour. Technol. 2019, 287, 121470.
- Westerholm, M.; Bettina, M.; Arthurson, V.; Anna, S. Changes in the Acetogenic Population in a Mesophilic Anaerobic Digester in Response to Increasing Ammonia Concentration. Microbes Environ. 2011, 26, 347–353.
- Pan, X.; Zhao, L.; Li, C.; Angelidaki, I.; Zhu, G. Deep insights into the network of acetate metabolism in anaerobic digestion: Focusing on syntrophic acetate oxidation and homoacetogenesis. Water Res. 2021, 190, 116774.
- Wang, D.; Duan, Y.; Yang, Q.; Liu, Y.; Ni, B.J.; Wang, Q.; Zeng, G.; Li, X.; Yuan, Z. Free ammonia enhances dark fermentative hydrogen production from waste activated sludge. Water Res. 2018, 133, 272–281.
- Hendriksen, H.V.; Ahring, B.K. Effects of ammonia on growth and morphology of thermophilic hydrogen-oxidizing methanogenic bacteria. FEMS Microbiol. Ecol. 1991, 85, 241–245.
More
