Immunological memory is fundamental to maintain immunity against re-invading pathogens. It is the basis for prolonged protection induced by vaccines and can be mediated by humoral or cellular responses - the latter largely mediated by T cells. Memory T cells belong to different subsets with specialized functions and distributions within the body. They can be broadly separated into circulating memory cells, which pace the entire body through the lymphatics and blood, and tissue-resident memory T (TRM) cells, which are constrained to peripheral tissues. Retained in the tissues where they form, TRM cells provide a frontline defense against reinfection. Here, we review this population of cells with specific attention to the liver, where TRM cells have been found to protect against infections, in particular those by Plasmodium species that cause malaria.
- resident memory T cells
- liver
1. Introduction
-
Introduction
The successful containment of infections relies on the speed with which immune responses of sufficient intensity are mounted. Immunological memory enables the long-term maintenance of a small fraction of those cells that responded to and resolved an earlier infection. The number of specific memory T cells generated after an infection, while declining over time, is generally larger than that of naïve T cells of the same specificities [1]. In addition, memory T cells display an enhanced antigen sensitivity, requiring lower levels of antigen for activation [2]. Memory T cells thus respond more rapidly and potently to pathogen invasion, and can exert efficient protection, potentially lifelong, against previously encountered infections. Different subsets of memory CD8+ T cells have been identified on the basis of their migratory properties, e.g., circulatory memory T cells and resident memory T cells (TRM cells). The latter have recently emerged as important mediators of protection in peripheral organs, a common point of entrance of pathogens, by inducing rapid and local responses upon antigen recall [3]. By combining transcriptional and phenotypic features with different approaches to investigate residency, studies have identified TRM cells in various disease models and within several tissue settings, including the liver. Importantly, strategies have been devised to favour the formation of TRM cells through vaccination, achieving promising results, for example, in the case of herpes virus infection in the mucosa of the female genital tract [4] and Plasmodium infection of the liver [5][6][7][8][9].
The liver is essential for the maintenance of homeostasis and is central to many metabolic and immunological processes. Hepatic functions are tightly regulated; and disturbances that lead to liver diseases such as microbial infections, chronic inflammation or cancer can result in death. The liver is also the target of certain pathogens, such as Plasmodium, Leishmania, or Listeria, which infect and develop in this organ during stages of their life cycles. Given the highly protective capacity of memory T cells, and in particular of TRM cells, studying the biology of these cells may aid the development of prophylactic and therapeutic strategies against life-threatening conditions associated with organ damage or infection.
2. Resident Memory T Cells
-
Resident Memory T Cells
As they are an essential first line of defense against pathogen invasion in most tissues, TRM cells have become a major focus of T cell research throughout the last decade. They have been identified in virtually all organs in mice [10][11] and humans [12] including lymphoid and non-lymphoid tissues (Table 1). While we will focus on CD8+ TRM cells in this review, TRM cells can derive from both CD4+ and CD8+ T cells.
Identification of cell surface markers that can clearly distinguish TRM cells from other memory T cell subsets in both mouse and human tissues is complicated by the fact that no single marker associated with TRM cells is exclusive to this cell subset. In the following table we try to summarize the expression of the canonical markers used to define CD8+ TRM cells in diverse murine and human organs.
Table 1.
Expression of the canonical markers used to define CD8
+
T
RM cells in diverse murine and human organs.
cells in diverse murine and human organs.
|
Organs |
Expression of Canonical Markers (CD69, CD103, CD49a and CXCR6) |
|
|
Mice |
Humans |
|
|
Intestine, Gut |
CD69+ CD103+/− CD49a+ CXCR6+ |
CD69+ CD103+ |
|
Skin |
CD69+ CD103+/− CD49a+ CXCR6+ |
CD69+ CD103+/− CD49a+/− |
|
Lungs |
CD69+ CD103+ CD49a+ CXCR6+/- |
CD69+ CD103+ CD49a+ CXCR6+ |
|
Female reproductive tract |
CD69+/− CD103+/− |
CD69+ CD103+ (transcriptomic profiling is yet to be determined) |
|
Salivary glands |
CD69+/− CD103+/− CD49a+ |
CD69+ CD103+/− |
|
Lymphoid organs (Spleen, lymph nodes, tonsil) |
CD69+ CD103− CD49a+ |
CD69+ CD103+/− CD49a- |
|
Liver |
CD69+ CD103− CD49a+ CXCR6+ |
CD69+ CD103+/− CXCR6+ |
|
Kidneys |
CD69+/− CD103- |
CD69+ CD103+/− CD49a+/− CXCR6+/− |
|
Pancreas |
CD69+/− CD103+/− |
CD69+ CD103+ CD49a+ CXCR6+ |
|
Brain |
CD69+ CD103+/− |
CD69+ CD103+/− CD49a+ CXCR6+/− |
|
Organs |
Expression of Canonical Markers (CD69, CD103, CD49a and CXCR6) |
|
|
Mice |
Humans |
|
|
Intestine, Gut |
CD69+ CD103+/− CD49a+ CXCR6+ |
CD69+ CD103+ |
|
Skin |
CD69+ CD103+/− CD49a+ CXCR6+ |
CD69+ CD103+/− CD49a+/− |
|
Lungs |
CD69+ CD103+ CD49a+ CXCR6+/- |
CD69+ CD103+ CD49a+ CXCR6+ |
|
Female reproductive tract |
CD69+/− CD103+/− |
CD69+ CD103+ (transcriptomic profiling is yet to be determined) |
|
Salivary glands |
CD69+/− CD103+/− CD49a+ |
CD69+ CD103+/− |
|
Lymphoid organs (Spleen, lymph nodes, tonsil) |
CD69+ CD103− CD49a+ |
CD69+ CD103+/− CD49a- |
|
Liver |
CD69+ CD103− CD49a+ CXCR6+ |
CD69+ CD103+/− CXCR6+ |
|
Kidneys |
CD69+/− CD103- |
CD69+ CD103+/− CD49a+/− CXCR6+/− |
|
Pancreas |
CD69+/− CD103+/− |
CD69+ CD103+ CD49a+ CXCR6+ |
|
Brain |
CD69+ CD103+/− |
CD69+ CD103+/− CD49a+ CXCR6+/− |
3. Liver TRM cell identification
-
Liver TRM cell identification
Malaria is a major infectious disease caused by Plasmodium parasites. In their vertebrate host, parasites first develop in the liver for a short period of time, where they infect hepatocytes, before being released into the bloodstream to cause blood-stage infection, which leads to disease symptoms. Early evidence supporting the existence of resident memory T cells in the liver came from studies investigating the role of CD8+ T cells against the liver-stage of Plasmodium. These studies identified a long-lasting population of memory CD8+ T cells present in the liver and absent in the spleen of mice vaccinated with radiation-attenuated Plasmodium sporozoites (the infectious stage transmitted by the mosquito) [13]. Vaccinated mice were protected against Plasmodium sporozoite challenge for more than 6 months [13]. Later reports revealed that a subpopulation of memory CD8+ T cells associated with the liver, but absent from the circulation, expressed high levels of CXCR6, CXCR3, and CD69 [5][14], markers commonly displayed by TRM cells [15].
The presence of bona fide memory cells permanently residing in the liver was confirmed by parabiosis studies in mice systemically infected with LCMV or Plasmodium sporozoites [6][11]. Parabiosis requires the surgical union of the flank skin of two animals. This enables the mixing of blood between the parabionts, and thus evaluation of T cell migration from one animal to the other. Unlike circulating cells, which equilibrate between both animals, resident populations remain in the parabiont in which they originally formed. This technique has been extensively used to identify TRM cells in different murine tissues [11]. Although liver TRM cells are in constant contact with circulating blood [6], parabiosis studies have confirmed that these cells, counterintuitively, do not recirculate and can only be found in the livers of the immunized parabiont partner [6][11].
Liver TRM cells were found to express a similar phenotypic and transcriptional signature to that of TRM cells previously identified in the lung, skin, and gut [6][16]. Maintenance of liver TRM cells in mice relies on the expression of the transcription factor Hobit, and on basal levels of expression of Blimp1 [17]. These TRM cell signatures have been found in T cells from grafted or isolated human tissues, enabling the unequivocal identification of TRM cells in several human organs [18], including the liver [19][20]. As mentioned earlier, contrary to liver TRM cells in mice which express high levels of Hobit and low to intermediate levels of Blimp1 [17], human liver TRM cells are Hobitlow Blimp1high [19]. In a recent publication, a small proportion of donor cells were found in HLA-mismatched liver and allografts 11 years after transplant, demonstrating the resident nature and remarkable longevity of these cells [21].
4. Liver TRM cell location
-
Liver TRM cell location
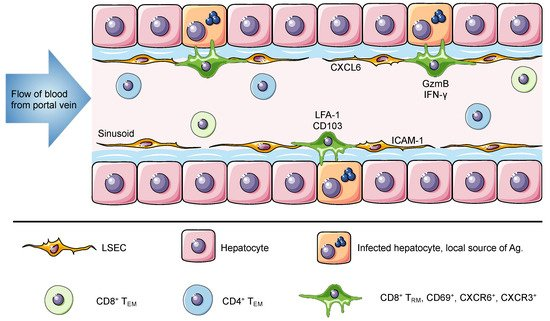
The liver is the recipient of both arterial and venous blood. The portal vein delivers large volumes of blood from the gastrointestinal tract and spleen to the liver. Once there, the blood flows through narrow vascular capillaries known as hepatic sinusoids, which reduce the flow rate and allow resident cells to interact with a vast variety of antigens and circulating cells [22]. The hepatic sinusoids are lined with liver sinusoidal endothelial cells that form a fenestrated thin layer that separates hepatocytes from circulating cells. These fenestrae grant lymphocytes in the blood direct access to the surface of hepatocytes for antigen recognition and effector function [23][24]. In contrast to TRM cells in most tissues, which are anatomically separated from the circulation, liver TRM cells are present within the sinusoids and are constantly exposed to the blood stream but are able to access antigen on tissue stroma through the fenestrated endothelium [6]. Intravital images shows that liver TRM cells, which display an ameboid shape, are uniquely located in the vasculature where they patrol the hepatic sinusoids at migration speeds more rapid than seen for skin TRM cells (Figure 1) [6][11][17].
Figure 1. The liver is a unique niche for tissue resident memory cells. The portal vein delivers antigen-rich blood from the gastrointestinal tract and spleen to the liver. This blood flows through the liver hepatic sinusoids lined with a thin layer of fenestrated liver sinusoidal endothelial cell (LSEC). Liver TRM cells are localized within the hepatic sinusoids, where they remain long-term and do not recirculate despite direct connection to the circulatory system and constant exposure to the blood. The expression of ICAM-1 and CXCL16 by LSEC can promote the retention of lymphocytes, through interactions with LFA-1 and CXCR6, respectively. Murine and human TRM cells in the liver express CD69, CXCR6, CXCR3 and high levels of LFA-1. Of note, human but not murine TRM cells express CD103. It has been suggested that this difference is associated with a broad versus a restricted expression of E-cadherin by human and murine hepatocytes, respectively. Intrahepatic lymphocytes including circulating and resident memory cells can access the surface of hepatocytes through LSEC fenestrae and exert effector functions. Using cytoplasmic protrusions, lymphocytes probe hepatocytes for the presence of antigen and can release factors such as GzmB and IFN-γ to promote hepatocyte killing. In murine studies, liver TRM cells can be generated through different vaccination strategies to confer protection against Plasmodium parasites and in humans they have been associated with disease control against HBV and HCV.
5. Liver TRM cell immune responses to infection
-
Liver TRM cell immune responses to infection
Murine studies have shown that liver TRM cells can confer efficient protection against liver-stage Plasmodium infection [6][9]. These studies have also demonstrated that substantial numbers of liver TRM cells are associated with higher levels of immunity to malaria, and depletion of these cells ablates protection [6][9]. Based on these results, several complex vaccinations strategies, aimed at trapping activated CD8+ T cells in the liver, have now successfully induced the formation of liver TRM cells in mice [6][7][8][9]. One vaccination strategy, prime-and-trap, is a single injection of a 3-component vaccine designed to prime Plasmodium-specific CD8+ T cells in the spleen and recruit them to the liver to form TRM cells via locally expressed antigen recognition and adjuvant-induced inflammation [6][9]. Another strategy, termed prime and target requires the administration of two components injected two weeks apart and uses a modified adenovirus for priming and either nanoparticles or a modified viral vector to target cells to the liver [7]. More recently, we have also used a glycoprotein-peptide vaccination strategy that utilizes NKT cell help to induce the formation of liver TRM cells [8]. In mice, vaccine-induced TRM cells patrol the liver sinusoids, form aggregates around infected hepatocytes and, based on expression of molecules such as GzmB, IFN-γ and TNF-α (Figure 1) [6][7], potentially exert infection control through direct lysis and/or cytokine-mediated mechanisms. Moreover, vaccination studies with attenuated Plasmodium sporozoites in non-human primates have found high frequencies of intrahepatic memory CD8+ T cells in protected subjects [25].
Importantly, in humans, liver TRM cells have been associated with disease control. For example, recent studies have investigated paired blood and liver samples from patients with chronic hepatitis B and hepatitis C virus infection and healthy volunteers to determine the role of liver TRM cells during viral infections [19][20]. Researchers found that human TRM cells in the liver express high levels of IL-2 and accumulate in larger numbers in the livers of infected patients compared to healthy patients. These studies also determined higher expression of GzmB and IFN-γ in HBV infected patients. Importantly, an inverse correlation between liver TRM frequencies and viral titers was observed, indicating that high numbers of specific liver TRM cells were associated with viral control [19]. However, accumulation of intrahepatic CD8+ CD103+ perforin+ T cells has been observed in cases of autoimmune hepatitis, particularly in indeterminate pediatric acute liver failure [26]. These findings suggest that liver TRM cells could also have a pathogenic function.
6. Conclusions
-
Conclusions
TRM cells are pivotal mediators of protective immune responses within tissues and have been identified in nearly all organs, including lymphoid, non-lymphoid and barrier tissues. They are loaded with effector molecules, including GzmB, perforin, IFN-γ, and TNF, and likely exert their function by the direct killing of targets, or by recruiting other immune cells. Several infection models have correlated the presence of TRM cells with pathogen and tumour control in tissues. Notably, in the liver, CD8+ TRM cells can mediate efficient control of liver-stage Plasmodium parasites, and likely, HBV and HCV infections. For this reason, TRM cells appear of particular interest in the course of vaccine development, especially for liver TRM cells for malaria vaccines. Further research unveiling the mechanisms for the formation and maintenance of TRM cells will facilitate the design of next generation TRM-based vaccines that realize the protective potential of these cells for unprecedented immunity against infections.
References
- Vandana Kalia; Surojit Sarkar; Rafi Ahmed; CD8 T-Cell Memory Differentiation during Acute and Chronic Viral Infections. Advances in Experimental Medicine and Biology 2010, 684, 79-95, 10.1007/978-1-4419-6451-9_7.
- Rashmi Kumar; María Ferez; Mahima Swamy; Ignacio Arechaga; María Teresa Rejas; Jose M. Valpuesta; Wolfgang Wa Schamel; Balbino Alarcón; Hisse M. Van Santen; Increased Sensitivity of Antigen-Experienced T Cells through the Enrichment of Oligomeric T Cell Receptor Complexes. Immunity 2011, 35, 375-387, 10.1016/j.immuni.2011.08.010.
- Thomas Gebhardt; Linda M Wakim; Liv Eidsmo; Patrick C Reading; William R Heath; Francis R Carbone; Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature Immunology 2009, 10, 524-530, 10.1038/ni.1718.
- Haina Shin; Akiko Iwasaki; A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 2012, 491, 463-467, 10.1038/nature11522.
- Sze-Wah Tse; Ian A. Cockburn; Hongwei Zhang; Alan L. Scott; Fidel Zavala; Unique transcriptional profile of liver-resident memory CD8+ T cells induced by immunization with malaria sporozoites. Genes & Immunity 2013, 14, 302-309, 10.1038/gene.2013.20.
- Daniel Fernandez-Ruiz; Wei Yi Ng; Lauren E. Holz; Joel Z. Ma; Ali Zaid; Yik Chun Wong; Lei Shong Lau; Vanessa Mollard; Anton Cozijnsen; Nicholas Collins; et al.Jessica LiGayle M DaveyYu KatoSapna DeviRoghieh SkandariMichael PauleyJonathan MantonDale I. GodfreyAsolina BraunSzun Szun TayPeck Szee TanDavid G. BowenFriedrich Koch-NolteBjörn RissiekFrancis R. CarboneBrendan S CrabbMireille LahoudIan A. CockburnScott N. MuellerPatrick BertolinoGeoffrey I. McFaddenIrina CaminschiWilliam R. Heath Liver-Resident Memory CD8 + T Cells Form a Front-Line Defense against Malaria Liver-Stage Infection. Immunity 2016, 45, 889-902, 10.1016/j.immuni.2016.08.011.
- Anita Gola; Daniel Silman; Adam A. Walters; Saranya Sridhar; Stefan Uderhardt; Ahmed M. Salman; Benedict R. Halbroth; Duncan Bellamy; Georgina Bowyer; Jonathan Powlson; et al.Megan BakerNavin VenkatramanIan PoultonEleanor BerrieRachel RobertsAlison M. LawrieBrian AngusShahid M. KhanChris J. JanseKatie J EwerRonald N. GermainAlexandra J. SpencerAdrian V. S. Hill Prime and target immunization protects against liver-stage malaria in mice. Science Translational Medicine 2018, 10, eaap9128, 10.1126/scitranslmed.aap9128.
- Lauren Holz; Yu Cheng Chua; Maria N. De Menezes; Regan J. Anderson; Sarah L. Draper; Benjamin J. Compton; Susanna T. S. Chan; Juby Mathew; Jasmine Li; Lukasz Kedzierski; et al.Zhongfang WangLynette BeattieMatthias H. EndersSonia GhilasRose MayThiago M. SteinerJoshua LangeDaniel Fernandez-RuizAna-Maria Valencia-HernandezTaryn L. OsmondKathryn J. FarrandRebecca SeneviratnaCatarina F. AlmeidaKirsteen M TullettPatrick BertolinoDavid G. BowenAnton CozijnsenVanessa MollardGeoffrey I. McFaddenIrina CaminschiMireille H LahoudKatherine KedzierskaStephen J. TurnerDale I. GodfreyIan F. HermansGavin F. PainterWilliam R. Heath Glycolipid-peptide vaccination induces liver-resident memory CD8+ T cells that protect against rodent malaria. Science Immunology 2020, 5, eaaz8035, 10.1126/sciimmunol.aaz8035.
- Ana Maria Valencia-Hernandez; Wei Yi Ng; Nazanin Ghazanfari; Sonia Ghilas; Maria N. De Menezes; Lauren E. Holz; Cheng Huang; Kieran English; Myo Naung; Peck Szee Tan; et al.Kirsteen M. TullettThiago M. SteinerMatthias H. EndersLynette BeattieYu Cheng ChuaClaerwen M. JonesAnton CozijnsenVanessa MollardYeping CaiDavid G. BowenAnthony W. PurcellNicole L. La GrutaJose A. VilladangosTania De Koning-WardAlyssa E. BarryWinfried BarchetIan A. CockburnGeoffrey I. McFaddenStephanie GrasMireille H. LahoudPatrick BertolinoRalf B. SchittenhelmIrina CaminschiWilliam R. HeathDaniel Fernandez-Ruiz A Natural Peptide Antigen within the Plasmodium Ribosomal Protein RPL6 Confers Liver TRM Cell-Mediated Immunity against Malaria in Mice. Cell Host & Microbe 2020, 27, 950-962.e7, 10.1016/j.chom.2020.04.010.
- Kerry A. Casey; Kathryn A. Fraser; Jason M. Schenkel; Amy Moran; Michael C. Abt; Lalit K. Beura; Philip J. Lucas; David Artis; E. John Wherry; Kristin Hogquist; et al.Vaiva VezysDavid Masopust Antigen-Independent Differentiation and Maintenance of Effector-like Resident Memory T Cells in Tissues. The Journal of Immunology 2012, 188, 4866-4875, 10.4049/jimmunol.1200402.
- Elizabeth M. Steinert; Jason M. Schenkel; Kathryn A. Fraser; Lalit K. Beura; Luke S. Manlove; Botond Z. Igyártó; Peter J. Southern; David Masopust; Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 2015, 161, 737-749, 10.1016/j.cell.2015.03.031.
- Taheri Sathaliyawala; Masaru Kubota; Naomi Yudanin; Damian Turner; Philip Camp; Joseph J. C. Thome; Kara L. Bickham; Harvey Lerner; Michael Goldstein; Megan Sykes; et al.Tomoaki KatoDonna L. Farber Distribution and Compartmentalization of Human Circulating and Tissue-Resident Memory T Cell Subsets. Immunity 2013, 38, 187-197, 10.1016/j.immuni.2012.09.020.
- Mimi Guebre-Xabier; Robert Schwenk; Urszula Krzych; Memory phenotype CD8+ T cells persist in livers of mice protected against malaria by immunization with attenuated Plasmodium bergheisporozoites. European Journal of Immunology 1999, 29, 3978-3986, 10.1002/(sici)1521-4141(199912)29:12<3978::aid-immu3978>3.3.co;2-s.
- Sze-Wah Tse; Andrea J. Radtke; Diego A. Espinosa; Ian A. Cockburn; Fidel Zavala; The Chemokine Receptor CXCR6 Is Required for the Maintenance of Liver Memory CD8+ T Cells Specific for Infectious Pathogens. The Journal of Infectious Diseases 2014, 210, 1508-1516, 10.1093/infdis/jiu281.
- Laura K. Mackay; Angus T. Stock; Joel Z. Ma; Claerwen M. Jones; Stephen J. Kent; Scott N. Mueller; William R. Heath; Francis R. Carbone; Thomas Gebhardt; Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proceedings of the National Academy of Sciences 2012, 109, 7037-7042, 10.1073/pnas.1202288109.
- Laura K Mackay; Azad Rahimpour; Joel Z Ma; Nicholas C Collins; Angus T Stock; Ming-Li Hafon; Javier Vega-Ramos; Pilar Lauzurica; Scott N Mueller; Tijana Stefanovic; et al.David C TscharkeWilliam R HeathMichael InouyeFrancis R CarboneThomas Gebhardt The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nature Immunology 2013, 14, 1294-1301, 10.1038/ni.2744.
- Laura K. Mackay; Martina Minnich; Natasja A. M. Kragten; Yang Liao; Benjamin Nota; Cyril Seillet; Ali Zaid; Kevin Man; Simon Preston; David Freestone; et al.Asolina BraunErica Wynne-JonesFelix M. BehrRegina StarkDaniel G. PellicciDale I. GodfreyGabrielle T. BelzMarc PellegriniThomas GebhardtMeinrad BusslingerWei ShiFrancis R. CarboneRené A. W. Van LierAxel KalliesKlaas P. J. M. Van Gisbergen Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016, 352, 459-463, 10.1126/science.aad2035.
- Brahma V. Kumar; Wenji Ma; Michelle Miron; Tomer Granot; Rebecca S. Guyer; Dustin J. Carpenter; Takashi Senda; Xiaoyun Sun; Siu-Hong Ho; Harvey Lerner; et al.Amy L. FriedmanYufeng ShenDonna L. Farber Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Reports 2017, 20, 2921-2934, 10.1016/j.celrep.2017.08.078.
- Laura J. Pallett; Jessica Davies; Emily J. Colbeck; Francis Robertson; Navjyot Hansi; Nicholas J.W. Easom; Alice R. Burton; Kerstin A. Stegmann; Anna Schurich; Leo Swadling; et al.Upkar S. GillVictoria MaleTuVinh LuongAmir GanderBrian R. DavidsonPatrick T.F. KennedyMala K. Maini IL-2high tissue-resident T cells in the human liver: Sentinels for hepatotropic infection. Journal of Experimental Medicine 2017, 214, 1567-1580, 10.1084/jem.20162115.
- Femke Stelma; Annikki De Niet; Marjan J. Sinnige; Karel A. Van Dort; Klaas P. J. M. Van Gisbergen; Joanne Verheij; Ester M. M. Van Leeuwen; Neeltje A. Kootstra; Hendrik W. Reesink; Human intrahepatic CD69 + CD8+ T cells have a tissue resident memory T cell phenotype with reduced cytolytic capacity. Scientific Reports 2017, 7, 1-10, 10.1038/s41598-017-06352-3.
- Laura J. Pallett; Alice R. Burton; Oliver E. Amin; Sergio Rodríguez‐Tajes; Amit A. Patel; Nekisa Zakeri; Anna Jeffery-Smith; Leo Swadling; Nathalie M Schmidt; Anna Baiges; et al.Amir GanderDominic YuDavid NasrallaFarid FroghiSatheesh IypeBrian R. DavidsonDouglas ThorburnSimon YonaXavier FornsMala K. Maini Longevity and replenishment of human liver-resident memory T cells and mononuclear phagocytes. Journal of Experimental Medicine 2020, 217, 317, 10.1084/jem.20200050.
- Mark W Robinson; Cathal Harmon; Cliona O'farrelly; Liver immunology and its role in inflammation and homeostasis. Cellular and Molecular Immunology 2016, 13, 267-276, 10.1038/cmi.2016.3.
- Luca G. Guidotti; Donato Inverso; Laura Sironi; Pietro Di Lucia; Jessica Fioravanti; Lucia Ganzer; Amleto Fiocchi; Maurizio Vacca; Roberto Aiolfi; Stefano Sammicheli; et al.Marta MainettiTiziana CataudellaAndrea RaimondiGloria Gonzalez-AseguinolazaUlrike ProtzerZaverio M. RuggeriFrancis V. ChisariMasanori IsogawaGiovanni SitiaMatteo Iannacone Immunosurveillance of the Liver by Intravascular Effector CD8 + T Cells. Cell 2015, 161, 486-500, 10.1016/j.cell.2015.03.005.
- Alessandra Warren; David G. Le Couteur; Robin Fraser; David G. Bowen; Geoffrey W. McCaughan; Patrick Bertolino; T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology 2006, 44, 1182-1190, 10.1002/hep.21378.
- Andrew S. Ishizuka; for the VRC 312 and VRC 314 Study Teams; Kirsten E Lyke; Adam DeZure; Andrea A Berry; Thomas L. Richie; Floreliz H Mendoza; Mary E Enama; Ingelise J Gordon; Lee-Jah Chang; et al.Uzma N SarwarKathryn L ZephirLaSonji A HolmanEric R JamesPeter F. BillingsleyAnusha GunasekeraSumana ChakravartyAnita ManojMinglin LiAdam J RubenTao LiAbraham G EappenRichard E StaffordNatasha K CTooba MurshedkarHope DecederfeltSarah H PlummerCynthia S HendelLaura NovikPamela J M CostnerJamie G SaundersMatthew B LaurensChristopher V PloweBarbara FlynnWilliam R WhalenJ P ToddJay NoorSrinivas RaoKailan Sierra-DavidsonGeoffrey M LynnJudith E EpsteinMargaret A KempGary A FahleSebastian A MikolajczakMatthew FishbaugherBrandon K SackStefan H. I. KappeSilas A DavidsonLindsey GarverNiklas K BjörkströmMartha C NasonBarney S. GrahamMario RoedererB. Kim Lee SimStephen L HoffmanJulie E. LedgerwoodRobert A Seder Correction: Corrigendum: Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nature Medicine 2016, 22, 692-692, 10.1038/nm0616-692c.
- Catherine Chapin; Thomas N. Burn; Tomas Meijome; Kathleen M. Loomes; Hector Melin-Aldana; Portia A Kreiger; Peter F. Whitington; Edward M. Behrens; Estella Alonso; Indeterminate pediatric acute liver failure is uniquely characterized by a CD103+ CD8+ T-cell infiltrate. Hepatology 2018, 68, 1087-1100, 10.1002/hep.29901.
- Andrew S. Ishizuka; for the VRC 312 and VRC 314 Study Teams; Kirsten E Lyke; Adam DeZure; Andrea A Berry; Thomas L. Richie; Floreliz H Mendoza; Mary E Enama; Ingelise J Gordon; Lee-Jah Chang; et al.Uzma N SarwarKathryn L ZephirLaSonji A HolmanEric R JamesPeter F. BillingsleyAnusha GunasekeraSumana ChakravartyAnita ManojMinglin LiAdam J RubenTao LiAbraham G EappenRichard E StaffordNatasha K CTooba MurshedkarHope DecederfeltSarah H PlummerCynthia S HendelLaura NovikPamela J M CostnerJamie G SaundersMatthew B LaurensChristopher V PloweBarbara FlynnWilliam R WhalenJ P ToddJay NoorSrinivas RaoKailan Sierra-DavidsonGeoffrey M LynnJudith E EpsteinMargaret A KempGary A FahleSebastian A MikolajczakMatthew FishbaugherBrandon K SackStefan H. I. KappeSilas A DavidsonLindsey GarverNiklas K BjörkströmMartha C NasonBarney S. GrahamMario RoedererB. Kim Lee SimStephen L HoffmanJulie E. LedgerwoodRobert A Seder Correction: Corrigendum: Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nature Medicine 2016, 22, 692-692, 10.1038/nm0616-692c.
- Catherine Chapin; Thomas N. Burn; Tomas Meijome; Kathleen M. Loomes; Hector Melin-Aldana; Portia A Kreiger; Peter F. Whitington; Edward M. Behrens; Estella Alonso; Indeterminate pediatric acute liver failure is uniquely characterized by a CD103+ CD8+ T-cell infiltrate. Hepatology 2018, 68, 1087-1100, 10.1002/hep.29901.