The reconstruction or repair of oral and maxillofacial functionalities and aesthetics is a priority for patients affected by tooth loss, congenital defects, trauma deformities, or various dental diseases. Therefore, in dental medicine, tissue reconstruction represents a major interest in oral and maxillofacial surgery, periodontics, orthodontics, endodontics, and even daily clinical practice. The current clinical approaches involve a vast array of techniques ranging from the traditional use of tissue grafts to the most innovative regenerative procedures, such as tissue engineering. In recent decades, a wide range of both artificial and natural biomaterials and scaffolds, genes, stem cells isolated from the mouth area (dental follicle, deciduous teeth, periodontal ligament, dental pulp, salivary glands, and adipose tissue), and various growth factors have been tested in tissue engineering approaches in dentistry, with many being proven successful. However, to fully eliminate the problems of traditional bone and tissue reconstruction in dentistry, continuous research is needed.
- regenerative medicine
- regenerative dentistry
- tissue engineering
- stem cells
- biomaterials
- scaffolds
1. Introduction
The traditional standard techniques based on replacing missing or deteriorated tissue with autologous grafts from living donors or even cadavers are still used in dentistry as well as in other medical fields, despite their disadvantages, such as risk of infections and rejection following the transplantation procedure. An innovative alternative is provided by regenerative medicine, which aims to regenerate, repair, or replace tissues and to ensure restoration of their impaired function by combining tissue engineering with the self-healing ability of humans. In vitro engineering of tissues and organs involves the emerging field of biotechnology in a multidisciplinary approach together with medicine, materials science, cell and molecular biology, bioengineering, and genetics [1].
Tissue engineering is a term associated with regenerative medicine and is distinct in its focus on aspects regarding the engineering and manufacturing of replacement tissue, but regenerative medicine and tissue engineering are often treated as a single field of interest in the literature. Tissue engineering aims to create functional tissue or even organs using patients’ own cells, offering an alternative method to grafts or transplants. This approach is being increasingly used in dental and maxillofacial reconstruction medicine, providing a new option for the reconstruction of teeth, periodontium, bones, oral mucosa, conjunctiva, skin, temporomandibular joint, both bone and cartilage as well as nerves, muscles, tendons, and blood vessels of the oral and maxillofacial area [2].
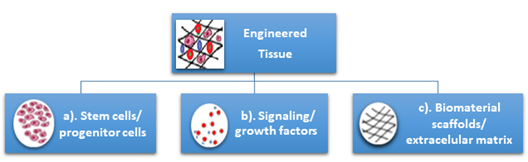
Tissue engineering can be used to regenerate tissue for specific defects, which represents a major advantage compared with other current treatments which have numerous disadvantages for patients like loss of sensorial and motor functionalities of craniofacial structures due to prosthetic alloplastic materials, high risk of infection, inflammation, requirement for lifelong immunosuppression, or unpredictable compatibility with the donor in the case of autologous grafts. Additionally, the unlimited available bioengineered resources do not require immunosuppression [3]. Tissue engineering is classically based on three pillars: (a) the cells (stem cells/progenitor cells), responsible for synthesizing the new tissue matrix; (b) the signaling/growth factors necessary to promote and facilitate the functionalities; (c) the biomaterial scaffolds, necessary for cell differentiation, multiplication, and biosynthesis, that act as an extracellular matrix (ECM) (Figure 1).
Figure 1. Classical pillars of tissue engineering: (a) the cells (stem cells/progenitor cells), (b) the signaling/growth factors, (c) the biomaterial scaffolds/extracellular matrix.
Cells communicate with their environment using different components to regenerate tissues by combining human cells with specific scaffold biomaterials. The biomaterial scaffolds provide templates for tissue regeneration and guide new tissues in their growth [4][5]. A successful approach in tissue engineering and regeneration implies that the combination of these three principles must be able to replace the damaged tissue and enable its function similarly to the original tissue or must be able to stimulate regeneration of the original tissue [6][7]. Several kinds of cells have been used in tissue engineering and regenerative medicine as reported in clinical studies, including stem cells, fibroblasts, chondrocytes, and keratinocytes originating from the same patient, another human, or animals [8].
The aim of this narrative review article is to approach this broad-spectrum subject in view of the literature from recent years specifically on the topic of potential orofacial stem cell usage in regenerative dentistry, both for hard and soft tissues. A large literature survey was performed on this topic in free-access digital archives of full-text articles (PubMed, Medline, Web of Science, and Google Scholar), with articles published between 2010–2020 being considered. More than 300 articles were referenced, with over 50% published in the last five years. The keywords used for searching were “regenerative dentistry”, “tissue engineering”, and “orofacial stem cells”. A specific search was performed to identify clinical studies involving the application of dental stem cells for tissue regeneration in endodontics, periodontics, and maxillofacial surgery.
2. Stem Cells, Biomaterials, and Scaffolds for Oral Tissue Engineering and Regeneration—Types, Sources, and Technologies
2.1. Orofacial Stem Cells
Stem cells (SCs) are defined as primitive, unspecialized, and pluripotent cells of the human body characterized by two major properties: production of other new stem cells and multidirectional differentiation into cells with a specific functionality, such as bone cells, skin cells, and blood cells [8][9]. Their presence was first reported in bone marrow [10].
SCs have powerful potential in medicine; their study has revealed important information about the complex processes of human body development. Due to these abilities, SCs have attracted interest regarding their use in the regeneration, repair, and functionality improvement of degenerated or injured tissue using implants of engineered tissue as well as biohybrid organs. The strategies involving the use of stem cells for tissue regeneration can be optimized using bioactive scaffolds or by adding various growth factors. [11].
Considering their origin, physiological stem cells include embryonic stem cells (ESCs) from embryos and adult stem cells (ASCs) from adult tissue. Other types of stem cells are the perinatal stem cells, from amniotic fluid, and induced pluripotent stem cells (iPSCs) [12], obtained by transforming regular ASCs under genetic reprogramming. iPSCs, which are generated directly from a somatic cell, were pioneered by Yamanaka, in 2006. Shinya Yamanaka’s discovery was awarded with the 2012 Nobel Prize, jointly with Sir John Gurdon, who, in 1962, demonstrated that the specialization of cells is reversible. The immature cell nucleus in an egg cell of a frog was replaced with the nucleus from a mature intestinal cell. This modified egg cell developed into a normal tadpole, proving that the DNA of the mature cell still had all the information needed to develop all cells in the frog [13]. More than 40 years later, Shinya Yamanaka discovered how intact mature cells in mice could be reprogrammed to become PSCs, able to develop into all types of cells in the body, by introducing only a few genes [14][15][16].
The ESCs are present in the blastocyst and can be differentiated into all types of cells, and are therefore pluripotent. Various postnatal tissues present ASCs for their normal renewal as well as regeneration or injury healing. Recent research in tissue engineering and regenerative medicine has demonstrated that SCs can be widely used in dentistry, more so than synthetic materials because teeth are a rich source of SCs [17]. Mesenchymal stem cells (MSCs) are a type of ASC of great importance in regenerative medicine due to their responsibilities in tissue repair and growth, cell substitution, and wound healing due to physiological or pathological causes. MSCs can be isolated especially from bone marrow and adipose tissue, but also from other various human tissues like the placenta, amniotic fluid, liver, umbilical cord, synovial membrane, skin, muscle, and dental tissues [18].
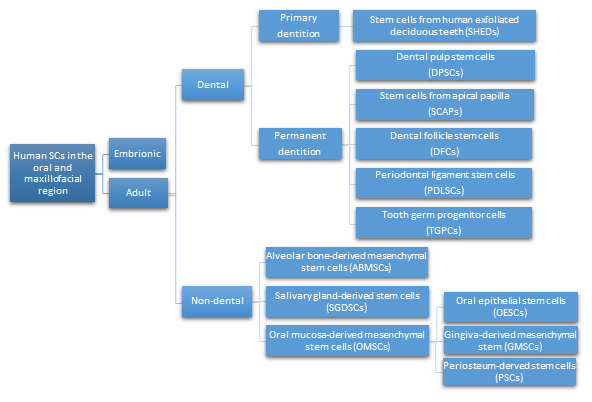
Different types of SCs obtained from oral and maxillofacial tissues, with similar in vitro properties as bone marrow-derived MSCs, are being defined as multipotent stromal cells. They are able to differentiate into different types of cells like chondrocytes, myocytes, osteoblasts, and adipocytes. Recently, the immunomodulatory properties of MSCs have been reported, which enable their clinical use in the treatment of inflammatory conditions [19]. Considering their location in the oral and maxillofacial region, the ASCs are grouped in two major categories: dental and non-dental [20] (Figure 2).
Figure 2. Types of human SCs in the oral and maxillofacial region.
The easy access, proliferation capacity, and multidirectional in vivo/in vitro differentiation makes orofacial SCs an important source of SCs for use in regenerative dentistry and medicine. Therefore, their potential clinical application in dentistry or other medical fields is diverse.
- Dental pulp stem cells (DPSCs), the first human dental MSCs found inside teeth, are considered a significant source for future regenerative procedures both in dental and general medical applications [21]. DPSCs are isolated from the dental pulp of primary or permanent teeth. Their high capacity for in vitro differentiation includes odontoblast, osteoblast, myoblast, adipocyte, dentin–pulp, cardiomyocyte, neuron-like cell, and hepatocyte-like cells, whereas in vivo, they are limited to only adipocytes, endotheliocytes, and myofibers [22][23].
- Periodontal ligament stem cells (PDLSCs), present on alveolar bone surfaces and the root, play a specific role in cementum or periodontal ligament (PDL) tissue regeneration. They are capable of giving rise to mesenchymal cell lineages to produce in vitro osteoblast-like cells, cementum tissue, Sharpey’s fibers, adipocytes, and collagen-forming cells [24].
- Stem cells from apical papilla (SCAPs) are mesenchymal They can be found within immature roots and isolated from the immature permanent apical papilla. SCAPs are good sources of and cause apexogenesis. They have a higher capacity to proliferate than DPSCs, being the first option for tissue regeneration. SCAPs represent a promising source of SCs, as they can differentiate into various lineages of cells, such as odontogenic, chondrogenic, osteogenic, adipogenic, neurogenic, and hepatogenic cells [25].
- Dental follicle stem cells (DFCs) are sourced from the dental follicle, which is loose connective tissue surrounding the developing tooth germ [17]. DFCs can differentiate osteoblast, cementoblast, alveolar bone, dentin-like tissues, PDL, cementum, adipocyte, chondrocyte, cardiomyocyte, and neuron-like cell. Their regenerative potential is highlighted by clinical applications in periodontal and neural tissue regeneration, tooth root regeneration, and bone defects [26][27].
- Tooth germ progenitor cells (TGPCs) are obtained from the dental mesenchyme of the human third molar germ in the late bell stage of tooth development. Studies on TGPCs have demonstrated their high proliferation activity and capacity to differentiation into adipogenic, chondrogenic, osteogenic, odontogenic, and neurogenic tissue [28][29]. In addition, TGPCs can differentiate into hepatocytes in vitro [30] and are able to form tube-like structures, possibly evidence of vascularization [31].
- Stem cells of human exfoliated deciduous teeth (SHEDs), obtained from exfoliated deciduous teeth, have higher proliferation capacity than DPSCs and the capability to differentiate into many more different body tissues than other types of SCs, including into adipocytes, osteoblasts, odontoblasts, neural cells, hepatocytes, and endothelial cells. SHEDs have a high proliferation capacity, high multipotency, immunosuppressive ability, and minimal risk of oncogenesis. [32]. The major disadvantage of SHEDs is that an incomplete pulp-dentin-like complex is formed in vivo.
- Alveolar bone-derived mesenchymal stem cells (ABMSCs), isolated from the human alveolar bone, are a more convenient tissue source of MSCs and have the ability of multipotent differentiation into osteoblasts, adipocytes, and chondroblasts. In addition, they can induce ectopic bone formation in vivo.
- Salivary gland-derived stem cells (SGDSCs) are isolated from human salivary glands. The regeneration of salivary gland function with SGDSCs is still being investigated, though certain studies have already concluded that progenitor cells isolated from stromal tissue can be guided to differentiate into osteoblasts, chondrocytes, and adipocytes [33].
- Oral mucosa-derived mesenchymal stem cells (OMSCs), include oral epithelial stem cells (OESCs), gingiva-derived mesenchymal stem cells (GMSCs), and periosteum-derived stem cells (PSCs). SCs within the mucosa lining the oral cavity can be isolated from normal or inflamed gingiva, from attached and free gingiva, and from hyperplastic gingiva. OMSCs can differentiate into different mesenchymal lineages and have immunomodulatory properties [33]
2.2. Biomaterials and Scaffolds for Oral Tissue Engineering
In dental tissue regeneration, scaffolds and biomaterials are essential elements. They are used as attachment sites for regenerative cells from the surrounding tissues, as a template for tissue regeneration, as a source of implantable odontogenic cells with the capability to differentiate required cell type, and as bioactive molecules, especially growth factors that intensify the regenerative capability [34][35].
Biomaterials, natural or synthetic, alive or lifeless, are being defined as materials that interact with biological systems. They are often used in medical applications to augment or replace a natural
function. Based on their biocompatibility, biomaterials are classified as bioactive, biotolerant, biodegradable, and bioinert [36].
Bioactive materials, by stimulating the biological response, may lead to osteogenesis by making strong chemical bonds. They are being classified into osteoconductive (hydroxyapatite and β-tricalcium phosphate), which stimulate bone growth along the surface, and osteoproductive (bioactive glasses), which are capable of stimulating the growth of new bone away from the bone/implant interface [36].
Biotolerant materials (polymers and most metals) are being well accepted by the host, but separated from the host tissue by the formation of a fibrous tissue, which is induced by the release of ions, corrosion products, and chemical compounds from the implant.
Biodegradable materials (polymers, such as polyglycolic and polylactic acids, and their co-polymers [37], ceramics as calcium phosphates [38], and magnesium) as biodegradable metal dissolve in contact with body fluids, the dissolution products being eliminated via the kidneys, without noticeable effects to the host. Biodegradable materials are used commonly used for surgical sutures, tissues in growth materials, and controlled drug release .
Bioinert materials (titanium and its alloys) are stable in the human body, and do not react with body fluids or tissues. Generally, bioinert materials are encapsulated by fibrous tissues, similar to biotolerant materials; however, in certain situations, they can develop structural and functional connection with the adjacent bone [39].
The most common approach in tissue engineering involves seeding cells onto a biomaterial matrix using a scaffold.
A wide variety of biomaterials, such as natural organic, synthetic organic, or even inorganic materials, is used for regeneration in oral and maxillofacial area, each of them having advantages and disadvantages. The natural organic materials include peptides (collagen or gelatin) and polysaccharides (alginate, chitosan, agarose). Frequently used synthetic organic materials include poly(lactic acid) (PLA), poly(caprolactone) (PCL), poly(lactic-co-glycolic acid) (PLGA), and poly(glycolic acid) (PGA).
The most commonly used inorganic materials are bioactive ceramics which include glasses or calcium phosphates (hydroxyapatite, β-tricalciumphosphate), which have been extensively studied as bone replacement materials, and cementitious systems of calcium phosphate or calcium silicate [40].
Bioactive ceramics are strongly chemically bonded with bone tissues via chemical reactions [40]. Hydroxyapatite (HA), bioactive and non-degradable, is characterized by chemical and structural similarity to bone minerals. β-tricalcium phosphate also has a chemical composition similar to bone,
and has higher in vivo rates of biodegradation compared to hydroxyapatite. The degradable bioactive ceramics are characterized by gradually degradation, in order to assist as scaffolds or replace the host tissue [40].
Polymers have been widely studied for medical applications, including bone tissue engineering [41]. From a biomedical perspective, polymers and co-polymers can be divided into two classes, biodegradable and biotolerant.
Biodegradable polymers, synthetic and natural, are suitable for additive manufacturing of scaffolds for tissue engineering [42]. The degradation of polymers, enzymatical or hydrolytical, is of most importance for this application. Natural polymers (chitosan, alginate, collagen, gelatin), frequently used as bioinks, are subject to enzymatic degradation, due to the microorganisms present in the biological environment [43].
The rate of enzymatic degradation varies upon the availability and concentration of respective enzymes. Hydrolytical degradation is related to synthetic polymers, and involves cleavage of hydrolytically sensitive bonds in the polymer, with consequent bulk or surface erosion, important in determining the best choice for a certain application [44].
Surface erosion offers several benefits for bone tissue engineering, such as retention of mechanical integrity, enhanced bone ingrowth, and ensures that the scaffold is gradually replaced by bone tissue [45].
PGA, PLA, PLGA, and PCL are hydrolytically degradable polymers [46].
PGA is usually used for short-term tissue engineering scaffolds and as fillers, because its rapid degradation and insolubility [47].
PLA, when mixed with glycolic acid, forms the copolymer PLGA, which is one of the most investigated degradable polymer for biomedical applications. Its great cell adhesion and proliferation properties recommend it as an excellent choice for tissue engineering [48][49].
Polymers can be processed to offer porous structures capable of facilitating the transportation of growth factors (nutrients as well as anabolites and catabolites) and are of interest due to their controllable degradation [41].
Recently, composite materials are being increasingly used due to their properties that result from the combination of both organic and inorganic elements. The most recent studies on this subject have considered the targeted and scaffold-assisted regeneration of enamel, dentin, and cementum.
An essential factor in tissue engineering is the scaffold. It offers a surface upon which cells adhere, multiply, thrive, and produce the ECM of proteins and saccharides that create the living tissue. Cells are expanded in culture and then transferred to the scaffold. The composition of the scaffold material and its internal architecture (dimensions of the struts, walls, pores, or channels) modulate and control the biological properties of the cells [50].
Generally, the scaffold materials must be biocompatible, biodegradable, porous, and without toxic metabolites. In particular, in dental regeneration, biomaterials must be suitable for the specific environment characteristics of the oral cavity considering pH, temperature, the presence of microorganisms, and the effect of mastication forces. To achieve these properties, most designed scaffolds deliberately mimic the structure of the natural ECM.
The number of suitable materials for fabricating scaffolds is limited by their biocompatibility, as they must accommodate the encapsulated cells and the recipient’s body. Because of poor biocompatibility, scaffolds can generate aggressive in vivo foreign-body reactions, necessitating the development of smart immunomodulatory biomaterials that ensure the tolerance of foreign scaffolds by the host or regulating the immunological microenvironments to ensure cell survival [49].
The behavior of cells after adhesion to the scaffold is affected by pore shape, volume, size, and geometry. Different pore sizes can affect the extracellular matrix. Porosity and interconnectivity are important for the ingrowth of surrounding tissues [51]. Open and interconnected pores allow oxygen and nutrients to be transported into the interior and eliminate the waste generated by cellular metabolism [52].
A wide range of advanced smart biomaterials and constructs with intelligent properties and functions have recently been developed to improve tissue repair and regeneration processes. Smart scaffolds incorporate bioactive molecules and nanoparticles and their physical and chemical properties are tailored as needed [53][54]. Their role is to improve the interactions with cells by enhancing the osteogenic differentiation for bone repair and to generate a better response to the surrounding environment [55] and include:
Smart scaffold constructs with stem cells for bone tissue engineering
- Biomimetic and bionic smart scaffolds, such as biomimetic porous PLGA microspheres coupled with peptides prepared to mimic the composition and structure of natural tissues [56].
- Immune-sensitive smart scaffolds, such as an amino-functionalized bioactive glass scaffold developed to investigate its effects on MSCs, bone marrow, and macrophages [57]. β-tricalcium phosphate has been used to coat Mg scaffolds, and modulate its detrimental osteoimmunomodulatory properties [58].
- Shape-memory smart scaffolds, such as bone morphogenetic protein2-loaded shape-memory porous nanocomposite scaffold, consisting of chemically crosslinked poly(ε-caprolactone) and hydroxyapatite nanoparticles, used for the repair of bone defects, displayed shape-memory recovery [59].
- Electromechanical-stimulus smart scaffolds. Piezoelectric poly(vinylidene fluoride-trifluoroethylene) (PVDF-TrFE) was fabricated into flexible, 3D fibrous scaffolds. These have the ability to stimulate MSCs differentiation and tissue formation [60]. An electrospun PVDF-TrFE fiber scaffold containing zinc oxide nanoparticles was able to promote the adhesion and proliferation of human MSCs and also enhance the blood vessel formation [61].
Smart drug delivery for bone tissue engineering
- Stimuli-responsiveness tunable drug delivery systems. These materials can change their properties as response to an endogenous and/or exogenous stimulus; thus, delivering the required amount of drug on-demand [62]. Polymers and hydrogels are used [63][64]. A highly porous, pH-responsive bacterial cellulose-g-poly(acrylic acidco-acrylamide) hydrogel was developed as an oral controlled-release drug delivery carrier [64]
- . A poly(ethylene glycol) hydrogel, loaded with drugs by β-eliminative linkers, demonstrated tunable capability in drug release [65]. Farnesol-loaded nanoparticles, composed of 2-(dimethylamino)ethyl methacrylate, butyl methacrylate, and 2-propylacrylic acid are characterized by a pH-responsive drug release capability [66].
- Smart multifunctional nanoparticle-based drug delivery systems: mesoporous silica nanoparticles, bone-forming peptide-1-laden MSNs encapsulated into arginine-glycine-aspartic acid-treated alginate hydrogel [67].
- Biomimetic drug delivery systems: hydrogels, liposomes, micelles, dendrimers, polymeric carriers, and nanostructures [68][69].
Smart biomaterials and constructs to promote dental and periodontal regeneration, such as bilayered PLGA/calcium phosphate constructs [70] and tri-layered nanocomposite hydrogel scaffold: alveolar bone phase of chitin-PLGA/nanobioactive glass ceramic (nBGC)/platelet-rich plasma derived growth factors, PDL phase of chitin-PLGA/fibroblast growth factor, and cementum phase of chitin-PLGA/nBGC/cementum protein 1.[71]
Smart dental resins that respond to pH to protect tooth structures, such as dental composites containing nanoparticles of amorphous calcium phosphate and tetracalcium phosphate [72].
Smart pH-sensitive materials selectively inhibit acid-producing bacteria, and include cationic poly(phenylene vinylene) derivative, pH-sensitive quaternary pyridinium salts, for which the antibacterial potency can be controlled by varying the pH [73][74].
Smart resins that modulate the oral biofilm composition: quaternary ammonium methacrylates such as 12-methacryloyloxy dodecyl pyridinium bromide, methacryloxylethyl cetyl dimethyl ammonium chloride, quaternary ammonium polyethylenimine, and dimethylaminododecyl methacrylate [75][76].
Smart tailoring of alkyl chain length in quaternary ammonium methacrylates to avoid drug resistance [77].
SCs are capable to differentiate into various cell phenotypes based on their lineage and exposure to different environmental stimuli, such as ECM, growth factors, hypoxia, etc. [78]. The growth factor, usually a secreted protein or a steroid hormone, stimulates wound healing, cell proliferation, and occasionally cellular differentiation, and regulates various cellular processes. Cytokines and hormones bind to specific receptors on the surface of the target cells. Growth factors typically act as signaling molecules between cells, thus promoting cell differentiation and maturation [79][80].
The authors experience related to the subject includes tetracycline loaded collagen-carboxymethylcellulose/hydroxyapatite ternary composite materials [81], antiseptic composite materials containing silver nanoparticles, based on collagen, hydroxyapatite, and collagen/hydroxyapatite [82], collagen matrices with lidocaine [83], bone regeneration using synthetic HA, with high porosity and surface area for osteointegration [84][85][86][87].
2.3. Additive Manufacturing Technologies for Oral Tissue Engineering
Continuous development of manufacturing technologies enable printing of biofunctional scaffolds similar to the ECM, acting as a microenvironment for cell adhesion, proliferation, and differentiation [88][89].
The additive manufacturing (3D printing) of biomaterials offers promising future perspectives for the field of biomedical engineering [90], especially in regard to patient-specific clinical applications.
Additive manufacturing techniques for medical and tissue engineering purposes can be classified as: techniques which involve printing of live cells along with other materials (3D bioprinting) [91], and non-cellular fabrication techniques.
3D bioprinting, based on the layer-by-layer precise positioning of biological constituents, biochemicals and living cells, facilitates on-demand “printing” of cells, tissues and organs [92][93] for regenerative medicine purposes [94]. Utilizing diverse bioprinting techniques, tissue-engineered constructs can be tailored to obtain desired structures and properties [95][96].
Inkjet bioprinting functions by depositing small ink droplets into a predetermined location. It can be driven by thermal or piezoelectric actuation [97]. In thermal technology, heat-generated, the inflated bubble forces the ink out of the narrow nozzle and onto the substrates. In piezoelectric technology, drops are generated in absence of heat, by the transient pressure from the piezoelectric actuator. The droplets remain directional with regular and equal size [98], but, if used too frequently, this technology can cause damage to the cell membrane and cell lysis.
Laser-based bioprinting consists of a pulsed laser source, a ribbon, and a receiving substrate. The biological material, in liquid form, is irradiated by the laser, evaporates, and reaches the receiving substrate as droplets. Laser-based bioprinting enables high-resolution printing of biological material such as cells, DNA, and peptides [99]. Its drawback is that the use of the pulsed laser source may result in compromised cell viability [100].
Stereolithography bioprinting uses a photo-crosslinking light source to obtain desired patterns. It is highly tunable and prints in a layer-by-layer manner, the bioink from the reservoir being transferred to a movable platform [101].
Pressure-assisted bioprinting uses biomaterials in form of solutions, pastes or dispersions. The material, in form of a filament, is extruded by pressure through a microneedle or a microscale nozzle orifice [102].
Bioink printability has an important role in the fabrication process [103][104]. Besides being biocompatible and biodegradable, bioinks should be deformable and flowable [102]. After printing, the bioink should be stable in order to maintain shape and architecture of the design model [105].
The components of the bioink are polymers, ceramics, hydrogels, and composites, currently used in tissue engineering [106]. Hydrogel inks are much more attractive as bioprinting materials, compared to polymers and ceramics have received much more attention, and novel ink formulations have been designed. [107]. Complex, functional, and biocompatible hydrogels can be fabricated using bioprinting technology. Adding different amounts of HA was attempted to a tunable alginate-gelatin hydrogel composite [108], human MSCs being subsequently mixed. Adding HA to the hydrogel resulted in enhanced mechanical properties, recommending it hard tissue reconstruction. No reduction in cell viability was detected [109]. The freeform reversible embedding of suspended hydrogels, a 3D bioprinting technique which deposits and crosslinks different kind of hydrogel inks, has been proven successfully [110].
An important concern when printing SCs—including ESCs, MSCs, and ASCs—is that their activity, including proliferation and pluripotency, may change during the process [111][112]. MSCs were successfully laser-printed for the construction of scaffold-free autologous grafts. The seed cells survived and maintained their ability to proliferate and continue differentiating into the osteogenic lineage [113].
Non-cellular additive manufacturing techniques include (Table 1):
The powder bed fusion methods which use either electron beam or laser to selectively consolidate material powder. The techniques involve spreading material powder over the previous layers, melting and fusing it [114].
The binder jetting technique is similar to the powder bed fusion technique and utilizes material powder that is spread over previous layers. Unlike powder bed fusion, this technique uses a binder as an adhesive for its consolidation [115][116].
The fused deposition modeling technique is based on the extrusion of heated polymer wires through a nozzle tip. The polymer rods are deposited and arranged in a layer by layer fashion [117].
The material jetting technique uses a liquid photopolymer resin that is light-cured. Similar to the material extrusion technique, the material is deposited from a nozzle and cured, defining a cross section. Individual cross sections are consolidated in a layer by layer fashion as the building platform moves in the vertical direction [118].
The vat polymerization technique uses a vat of liquid photopolymer resin, deposited in a layer by layer fashion. The build platform moves (depending on the position of the light source) to create additional layers on top of the previous [119].
These techniques all have their pros and cons and can process different types of biomaterials [120] (Table 1).
Table 1. Additive manufacturing methods of biomaterials for oral tissue engineering
|
Biomaterial |
Type |
Fabrication Method |
Application |
Reference |
|
Hydroxyapatite |
Bioactive/non-degradable ceramic |
Vat polymerization; powder bed fusion; fused deposition; binder jetting |
Bone tissue engineering |
|
|
Bio glass |
Bioactive ceramic |
Vat polymerization |
Bone tissue engineering |
[126] |
|
Calcium silicate |
Bioactive ceramic |
Powder bed fusion |
Tissue engineering |
[127] |
|
β-tricalcium phosphate |
Bioactive/ biodegradable ceramic |
Binder jetting; vat polymerization; fused deposition |
Bone tissue engineering |
|
|
Polycaprolactone |
Biodegradable polymer |
Powder bed fusion; fused deposition |
Bone tissue engineering; cartilage tissue engineering |
|
|
Poly(lactic acid) |
Biodegradable polymer |
Fused deposition |
Bone regeneration |
[137] |
|
Poly(lactic acid-co-glycolic acid) |
Biodegradable polymer |
Material jetting; fused deposition |
Tissue engineering |
References
- Tatullo, M.; Marrelli, M.; Paduano, F. The regenerative medicine in oral and maxillofacial surgery: The most important innovations in the clinical application of mesenchymal stem cells. Int. J. Med. Sci. 2015, 12, 72–77, doi:10.7150/ijms.10706.
- Rai, R. Tissue engineering: Step ahead in maxillofacial reconstruction. J. Int. Oral Health 2015, 9, 138–142.
- Borrelli, M.R.; Hu, M.S.; Longaker, M.T.; Lorenz, H.P. Tissue engineering and regenerative medicine in craniofacial reconstruction and facial aesthetics. J. Craniofac. Surg. 2020, 31, 15–27, doi:10.1097/scs.0000000000005840.
- Upadhyay, R.K. Role of Biological Scaffolds, Hydro Gels and Stem Cells in Tissue Regeneration Therapy. Adv. Tissue Eng. Regen. Med. Open Access 2017, 2, 121–135, doi:10.15406/atroa.2017.02.00020.
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 31, doi:10.1038/s41413-018-0032-9.
- Guan, X.; Avci-Adali, M.; Alarcin, E.; Cheng, H.; Kashaf., S.S.; Li, Y.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017, 12, 1600394, doi:10.1002/biot.201600394.
- Gao, Z.H.; Hu, L.; Liu, G.L.; Wei, F.L.; Liu, Y.; Liu, Z.H.; Fan, Z.P.; Zhang, C.M.; Wang, J.S.; Wang, S.L. Bio-Root and Implant-Based Restoration as a Tooth Replacement Alternative. J. Dent. Res. 2016, 95, 642–649, doi:10.1177/0022034516639260.
- Har, A.; Park, J.C. Dental Stem Cells and Their Applications. Chin. J. Dent. Res. 2015, 18, 207–212, doi:10.3290/j.cjdr.a35143.
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 1–12, doi:10.1155/2016/4709572.
- Drela, K.; Stanaszek, L.; Nowakowski, A.; Kuczynska, Z.; Lukomska, B. Experimental strategies of mesenchymal stem cell propagation: Adverse events and potential risk of functional changes. Stem Cells Int. 2019, 2019, 7012692, doi:10.1155/2019/7012692.
- Zhou, T.; Pan, J.; Wu, P.; Huang, R.; Du, W.; Zhou, Y.; Wan, M.; Fan, Y.; Xu, X.; Zhou, X.; et al. Dental Follicle Cells: Roles in Development and Beyond. Stem Cells Int. 2019, 2019, 9159605, doi:10.1155/2019/9159605.
- The Nobel Prize in Physiology or Medicine—2012 Press Release. Available online: www.nobelprize.org/prizes/medicine/2012/press-release/ (accessed on 12 November 2020).
- Gurdon, J.B. The Developmental Capacity of Nuclei Taken from Intestinal Epithelium Cells of Feeding Tadpoles. J. Embryol. Exp. Morphol. 1962, 10, 622–640.
- Yamanaka, S. Patient-specific pluripotent stem cells become even more accessible. Cell Stem Cell 2010, 7, 1–2, doi:10.1016/j.stem.2010.06.009.
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872, doi:10.1016/j.cell.2007.11.019.
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676, doi:10.1016/j.cell.2006.07.024.
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 1–22, doi:10.1186/s13287-019-1165-5.
- Berebichez-Fridman, R.; Pablo, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells. Sultan Qaboos Univ. Med. J. 2018, 18, e264, doi:10.18295/squmj.2018.18.03.002.
- Cao, C.; Tarlé, S.; Kaigler, D. Characterization of the immunomodulatory properties of alveolar bone-derived mesenchymal stem cells. Stem Cell Res. 2020, 11, 102, doi:10.1186/s13287-020-01605-x.
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 2015, 33, 627–638, doi:10.1002/stem.1909.
- Pisciotta, A.; Carnevale, G.; Meloni, S.; Riccio, M.; De Biasi, S.; Gibellini, L. Human dental pulp stem cells (hDPSCs): Isolation, enrichment, and comparative differentiation of two sub-populations. BMC Dev. Biol. 2015, 15, 14, doi:10.1186/s12861-015-0065-x.
- Hollands, P.; Aboyeji, D.; Orcharton, M. Dental pulp stem cells in regenerative medicine. Br. Dent. J. 2018, 224, 747, doi:10.1038/sj.bdj.2018.348.
- Tsutsui, T.W. Dental Pulp Stem Cells: Advances to Applications. Stem Cells Cloning Adv. Appl. 2020, 13, 33–42, doi:10.2147/SCCAA.S166759.
- Sharpe, P.T. Dental mesenchymal stem cells. Development 2016, 143, 2273–2280, doi:10.1242/dev.134189.
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. BioMed Res. Int. 2019, 2019, 6104738, doi:10.1155/2019/6104738.
- Tian, Y.; Bai, D.; Guo, W.; Li, J.; Zeng, J.; Yang, L.; Jiang, Z.; Feng, L.; Yu, M.; Tian, W. Comparison of human dental follicle cells and human periodontal ligament cells for dentin tissue regeneration. Regen. Med. 2015, 10, 461–479, doi:10.2217/rme.15.21.
- Dave, J.R.; Tomar, G.B. Dental Tissue-Derived Mesenchymal Stem Cells: Applications in Tissue Engineering. Crit. Rev. Biomed. Eng. 2018, 46, 429–468, doi:10.1615/CritRevBiomedEng.2018027342.
- Paz, A.G.; Maghaireh, H.; Mangano, F.G. Stem Cells in dentistry: Types of intra- and extraoral tissue-derived stem cells and clinical applications. Stem Cells Int. 2018, 2018, 4313610, doi:10.1155/2018/4313610.
- Motwani, B.K.; Singh, M.; Kaur, G.; Singh, S.; Gangde, P.O. Stem cells: A new paradigm in dentistry. Stem Cells 2016, 2, 140, doi:10.4103/2155-8213.183764.
- Chalisserry, E.P.; Nam, S.Y.; Park, S.H.; Anil, S. Therapeutic potential of dental stem cells. J. Tissue Eng. 2017, 8, 1–17, doi:10.1177/2041731417702531.
- Somani, R.; Jaidka, S.; Bajaj, N.; Arora, S. Miracle cells for natural dentistry—A review. J. Oral Biol. Craniofac. Res. 2017, 7, 49–53, doi:10.1016/j.jobcr.2015.11.007.
- Taguchi, T.; Yanagi, Y.; Yoshimaru, K.; Zhang, X.Y.; Matsuura, T.; Nakayama, K.; Kobayashi, E.; Yamaza, H.; Nonaka, K.; Ohga, S.; et al. Regenerative medicine using stem cells from human exfoliated deciduous teeth (SHED): A promising new treatment in pediatric surgery. Surg. Today 2019, 49, 316–322, doi:10.1007/s00595-019-01783-z.
- Grawish, M.E. Gingival-derived mesenchymal stem cells: An endless resource for regenerative dentistry. World J. Stem Cells 2018, 10, 116–118, doi:10.4252/wjsc.v10.i9.116.
- Tonk, C.; Witzler, M.; Schulze, M.; Tobiasch, E. Mesenchymal Stem Cells. In Essential Current Concepts in Stem Cell Biology; Brand-Saberi, B., Ed.; Springer: Berlin, Germany, 2020; pp. 21–39.
- Baranova, J.; Büchner, D.; Götz, W.; Schulze, M.; Tobiasch, E. Tooth Formation: Are the Hardest Tissues of Human Body Hard to Regenerate? Int. J. Mol. Sci. 2020, 21, 4031; doi:10.3390/ijms2111403121(11).
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. 2020, 108, 1617–1633, doi:10.1002/jbm.a.36930.
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280, doi:10.1016/j.progpolymsci.2011.06.004.
- Xie, Y.; Chen, Y.; Sun, M.; Ping, Q. A mini review of biodegradable calcium phosphate nanoparticles for gene delivery. Curr. Pharm. Biotechnol. 2013, 14, 918–925, doi:10.2174/1389201014666131226145441.
- Bergmann, C.P.; Stumpf, A. Biomaterials. In Dental Ceramics: Microstructure, Properties and Degradation; Bergmann, C., Stumpf, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 9–13, doi:10.1007/978-3-642-38224-6_2.
- Ferrage, L.; Bertrand, G.; Lenormand, P.; Grossin, D.; Ben-Nissan, B. A review of the additive manufacturing (3DP) of bioceramics: Alumina, zirconia (PSZ) and hydroxyapatite. J. Aust. Ceram. Soc. 2017, 53, 11–20, doi:10.1007/s41779-016-0003-9.
- Liu, X.; Ma, P.X. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486, doi:10.1023/B:ABME.0000017544.36001.8e.
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798, doi:10.1016/j.progpolymsci.2007.05.017.
- Banerjee, A.; Chatterjee, K.; Madras, G. Enzymatic degradation of polymers: A brief review. Mater. Sci. Technol. 2014, 30, 567–573, doi:10.1179/1743284713Y.0000000503.
- Rey-Vinolas, S.; Engel, E.; Mateos-Timoneda, M. Polymers for bone repair. In Bone Repair Biomaterials, 2nd ed.; Pawelec, K.M., Planell, J.A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 179–197, doi:10.1016/B978-0-08-102451-5.00007-X.
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431, doi:10.1016/j.biomaterials.2006.01.039.
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. 2011, 49, 832–864, doi:10.1002/polb.22259.
- Maurus, P.B.; Kaeding, C.C. Bioabsorbable implant material review. Oper. Tech. Sports Med. 2004, 12, 158–160, doi:10.1053/j.otsm.2004.07.015.
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic)Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659, doi:10.3390/ijms15033640.
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid)micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380, doi:10.1007/s40005-019-00439-x.
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926, doi:10.1126/science.1226340. .
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part A 2015, 21, 486–497, doi:10.1089/ten.tea.2013.0545.
- Domingos, M.; Intranuovo, F.; Russo, T.; De Santis, R.; Gloria, A.; Ambrosio, L.; Ciurana, J.; Bartolo, P. The first systematic analysis of 3D rapid prototyped poly(epsilon-caprolactone) scaffolds manufactured through BioCell printing: The effect of pore size and geometry on compressive mechanical behaviour and in vitro hMSC viability. Biofabrication 2013, 5, 045004, doi:10.1088/1758-5082/5/4/045004.
- Motamedian, S.R.; Hosseinpour, S.; Ahsaie, M.G.; Khojasteh, A. Smart scaffolds in bone tissue engineering: A systematic review of literature. World J. Stem Cells 2015, 7, 657–668, doi:10.4252/wjsc.v7.i3.657.
- Kaigler, D.; Wang, Z.; Horger, K.; Mooney, D.J.; Krebsbach, P.H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J. Bone Min. Res. 2006, 21, 735–744, doi:10.1359/jbmr.060120.
- Khan, F.; Tanaka, M. Designing smart biomaterials for tissue engineering. Int. J. Mol. Sci. 2018, 19, 17, doi:10.3390/ijms19010017.
- Mittal, A.; Negi, P.; Garkhal, K.; Verma, S.; Kumar, N. Integration of porosity and bio-functionalization to form a 3D scaffold: Cell culture studies and in vitro degradation. Biomed. Mater. 2010, 5, 045001, doi:10.1088/1748-6041/5/4/045001.
- Zeng, D.; Zhang, X.; Wang, X.; Huang, Q.; Wen, J.; Miao, X.; Peng, L.; Li, Y.; Jiang, X. The osteoimmunomodulatory properties of MBG scaffold coated with amino functional groups. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1425–1435, doi:10.1080/21691401.2017.1369428.
- Chen, Z.; Mao, X.; Tan, L.; Friis, T.; Wu, C.; Crawford, R.; Xiao, Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials 2014, 35, 8553–8565, doi:10.1016/j.biomaterials.2014.06.038.
- Liu, X.; Zhao, K.; Gong, T.; Song, J.; Bao, C.; Luo, E.; Weng, J.; Zhou, S. Delivery of growth factors using a smart porous nanocomposite scaffold to repair a mandibular bone defect. Biomacromolecules 2014, 15, 1019–1030, doi:10.1021/bm401911p.
- Damaraju, S.M.; Shen, Y.; Elele, E.; Khusid, B.; Eshghinejad, A.; Li, J.; Jaffe, M.; Arinzeh, T.L. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials 2017, 149, 51–62, doi:10.1016/j.biomaterials.2017.09.024.
- Augustine, R.; Dan, P.; Sosnik, A.; Kalarikkal, N.; Tran, N.; Vincent, B.; Thomas, S.; Menu, P.; Rouxel, D. Electrospun poly(vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation. Nano Res. 2017, 10, 3358–3376, doi:10.1007/s12274-017-1549-8.
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323, doi:10.7150/thno.14858.
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Pillay, V. A Review of Injectable Polymeric Hydrogel Systems for Application in Bone Tissue Engineering. Molecules 2016, 21, 1580, doi:10.3390/molecules21111580.
- Mohd Amin, M.C.; Ahmad, N.; Pandey, M.; Jue Xin, C. Stimuli-responsive bacterial cellulose-g-poly(acrylic acid-co-acrylamide) hydrogels for oral controlled release drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 1340–1349, doi:10.3109/03639045.2013.819882.
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323, doi:10.1073/pnas.1215498110.
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404, doi:10.1021/nn507170s.
- Luo, Z.; Zhang, S.; Pan, J.; Shi, R.; Liu, H.; Lyu, Y.; Han, X.; Li, Y.; Yang, Y.; Xu, Z.; et al. Time-responsive osteogenic niche of stem cells: A sequentially triggered, dual-peptide loaded, alginate hybrid system for promoting cell activity and osteo-differentiation. Biomaterials 2018, 163, 25–42, doi:10.1016/j.biomaterials.2018.02.025.
- Sant, S.; Hancock, M.J.; Donnelly, J.P.; Iyer, D.; Khademhosseini, A. Biomimetic gradient hydrogels for tissue engineering. Can. J. Chem. Eng. 2010, 88, 899–911, doi:10.1002/cjce.20411.
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in drug delivery systems: A critical review. J. Control. Release 2017, 253, 97–109, doi:10.1016/j.jconrel.2017.03.026.
- Carlo Reis, E.C.; Borges, A.P.; Araújo, M.V.; Mendes, V.C.; Guan, L.; Davies, J.E. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials 2011, 32, 9244–9253, doi:10.1016/j.biomaterials.2011.08.040.
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Adv. Healthc. Mater. 2017, 6, 1601251, doi:10.1002/adhm.201601251.
- Weir, M.D.; Ruan, J.; Zhang, N.; Chow, L.C.; Zhang, K.; Chang, X.; Bai, Y.; Xu, H.H.K. Effect of calcium phosphate nanocomposite on in vitro remineralization of human dentin lesions. Dent. Mater. 2017, 33, 1033–1044, doi:10.1016/j.dental.2017.06.015.
- Li, L.; He, J.; Eckert, R.; Yarbrough, D.; Lux, R.; Anderson, M.; Shi ,W. Design and characterization of an acid-activated antimicrobial peptide. Chem. Biol. Drug Des. 2010, 75, 127–132, doi:10.1111/j.1747-0285.2009.00904.x.
- Yang, Y.; Reipa, V.; Liu, G.; Meng, Y.; Wang, X.; Mineart, K.P.; Prabhu, V.M.; Shi, W.; Lin, N.J.; He, X.; et al. pH-sensitive compounds for selective inhibition of acid-producing bacteria. ACS Appl. Mater. Interfaces 2018, 10, 8566–8573, doi:10.1021/acsami. 8b01089.
- Cheng, L.; Weir, M.D.; Zhang, K.; Wu, E.J.; Xu, S.M.; Zhou, X.; Xu, H.H. Dental plaque microcosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium. Dent. Mater. 2012, 28, 853–862, doi:10.1016/j.dental.2012.04.024.
- Wang, S.; Wang, H.; Ren, B.; Li, X.; Wang, L.; Zhou, H.; Weir, M.D.; Zhou, X.; Masri, R.M.; Oates, T.W.; et al. Drug resistance of oral bacteria to new antibacterial dental monomer dimethylaminohexadecyl methacrylate. Sci. Rep. 2018, 8, 5509, doi:10.1038/s41598-018-23831-3.
- Li, F.; Weir, M.D.; Xu, H.H. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938, doi:10.1177/0022034513502053.
- Li, L.; Zhu, Y.Q.; Jiang, L.; Peng, W.; Ritchie, H.H. Hypoxia Promotes Mineralization of Human Dental Pulp Cells. J. Endod. 2011, 37, 799–802, doi:10.1016/j.joen.2011.02.028.
- Huang, G.T.J.; Shagramanova, K.; Chan, S.W. Formation of Odontoblast-Like Cells from Cultured Human Dental Pulp Cells on Dentin In Vitro. J. Endod. 2006, 32, 1066–1073, doi:10.1016/j.joen.2006.05.009.
- Del Angel-Mosqueda, C.; Gutiérrez-Puente, Y.; López-Lozano, A.P.; Romero-Zavaleta, R.E.; Mendiola-Jiménez, A.; Medina-De la Garza, C.E.; Márquez, M.M.; De la Garza-Ramos, M.A. Epidermal growth factor enhances osteogenic differentiation of dental pulp stem cells in vitro. Head Face Med. 2015, 11, 29, doi:10.1186/s13005-015-0086-5.
- Rusu, L.C.; Nedelcu, I.V.; Albu, M.G.; Sonmez, M.; Voicu, G.; Radulescu, M.; Ficai, D.; Ficai, A.; Negrutiu, M.L.; Sinescu, C. Tetracycline Loaded Collagen/Hydroxyapatite Composite Materials for Biomedical Applications. J. Nanomater. 2015, 2015, 361969, doi:10.1155/2015/361969.
- Patrascu, J.M.; Nedelcu, I.A.; Sonmez, M.; Ficai, D.; Ficai, A.; Vasile, B.S.; Ungureanu, C.; Albu, M.G.; Andor, B.; Andronescu, E.; et al. Composite Scaffolds Based on Silver Nanoparticles for Biomedical Applications. J. Nanomater. 2015, 2015, 587989, doi:10.1155/2015/587989.
- Rusu, L.C.; Sinescu, C.; Negrutiu, M.L.; Ardelean, L.; Ogodescu, A.; Fabricky, M.; Petrescu, E.; Rominu, R.; Topala, F.; Rominu, M.; et al. Application for regenerative dentistry: The collagen matrices with lidocaine. In Computational Engineering in System Applications. In Proceedings of the International Conference on Energy, Environment, Economics, Devices, Systems, Communications, Computers, Iasi, Romania, 1–3 July 2011, pp. 60–64.
- Rusu, LC.; Manescu, A.; Negrutiu, M.L.; Sinescu, C.; Ardelean, S.; Hoinoiu, B.; Rominu, M. The Micro CT Evaluation of Different Types of Matrices in Rats Bone Augumentation. Key Eng. Mater. 2013, 587, 338–342, doi:10.4028/www.scientific.net/kem.587.338.
- Rusu, L.C.; Negrutiu, M.L.; Sinescu, C.; Hoinoiu, B.; Topala, F.I.; Duma, V.F.; Rominu, M.; Podoleanu, A.G. Time domain optical coherence tomography investigation of bone matrix interface in rat femurs. In Proceedings of the ISPDI 2013-Fifth International Symposium on Photoelectronic Detection and Imaging (SPIE 8914), Beijing, China, 25–27 June 2013; p. 89141 H, doi:10.1117/12.2036345.
- Rusu, L.C.; Negrutiu, M.L.; Sinescu, C.; Hoinoiu, B.; Zaharia, C.; Ardelean, L.; Duma, V.F.; Podoleanu, A.G. Different matrix evaluation for the bone regeneration of rats’ femours using time domain optical coherence tomography. In Proceedings of the Fifth International Conference on Lasers in Medicine: Biotechnologies Integrated in Daily Medicine (SPIE 8925), Timisoara, Romania, 19-21 September 2013; p. 89250V, doi:10.1117/12.2045849.
- Manescu, A.; Oancea, R.; Todea, C.; Rusu, L.C.; Mazzoni, S.; Negrutiu, M.L.; Sinescu, C.; Giuliani, A. On Long Term Effects of Low Power Laser Therapy on Bone Repair: A Demonstrative Study by Synchrotron Radiation-based Phase-Contrast Microtomography. Int. J. Radiol. Imaging Technol. 2016, 2, 010, doi:10.23937/2572-3235.1510010.
- Baumgartner, S.; Gmeiner, R.; Schönherr, J.A.; Stampfl, J. Stereolithography-based additive manufacturing of lithium disilicate glass ceramic for dental applications. Mater. Sci. Eng. C 2020, 116, 111180, doi:10.1016/j.msec.2020.111180.
- Jasiuk, I.; Abueidda, D.W.; Kozuch, C.; Pang, S.; Su, F.Y.; McKittrick, J. An Overview on Additive Manufacturing of Polymers. JOM 2018, 70, 275–283, doi:10.1007/s11837-017-2730-y.
- Mederle, N.; Marin, S.; Marin, M.M.; Danila, E.; Mederle, O.; Kaya, M.G.A.; Ghica, M.V. Innovative biomaterials based on collagen-hydroxyapatite and doxycycline for bone regeneration. Adv. Mater. Sci. Eng. 2016, 2016, 3452171, doi:10.1155/20163452171.
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284–289, doi:10.6515/ACS.201905_35(3).20181115A.
- Zadpoor, A.A.; Malda, J. Additive manufacturing of biomaterials, tissues, and organs. Ann. Biomed. Eng. 2017, 45, 1–11, doi:10.1007/s10439-016-1719-y.
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. B Eng. 2018, 143, 172–196, doi:10.1016/j.compositesb.2018.02.012.
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785, doi:10.1038/nbt.2958.
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Hou, W.; Tong, H.; Bai, S. 3D bioprinting technologies for hard tissue and organ engineering. Materials (Basel) 2016, 9, 802, doi:10.3390/ma9100802.
- Ji, X.; Zhu, H.; Zhao, L.; Xiao, J. Recent advances in 3D bioprinting for the regeneration of functional cartilage. Regen. Med. 2018,13, 73–87, doi:10.2217/rme-2017-0106.
- Nakamura, M.; Kobayashi, A.; Takagi, F.; Watanabe, A.; Hiruma, Y.; Ohuchi, K.; Iwasaki, Y.; Horie, M.; Morita, I.; Takatani, S. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 2005, 11, 1658–1666, doi:10.1089/ten.2005.11.1658.
- Saunders, R.E.; Gough, J.E.; Derby, B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials 2008, 29, 193–203, doi:10.1016/j.biomaterials.2007.09.032.
- Catros, S.; Fricain, J.C.; Guillotin, B.; Pippenger, B.; Bareille, R.; Remy, M.; Lebraud, E.; Desbat, B.; Amedee, J.; Guillemot, F. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nanohydroxyapatite. Biofabrication 2011, 3, 025001, doi:10.1088/1758-5082/3/2/025001.
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B. Laser-assisted cell printing: Principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine 2010, 5, 507–515, doi:10.2217/nnm.10.14.
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339, doi:10.1016/j.biomaterials.2012.09.048.
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271, doi:10.1186/s12967-016-1028-0.
- Theus, A.S.; Ning, L.; Hwang, B.; Gil, C.; Chen, S.; Wombwell, A.; Mehta, R.; Serpooshan, V. Bioprintability: Physiomechanical and Biological Requirements of Materials for 3D Bioprinting Processes. Polymers (Basel) 2020, 12, 2262, doi:10.3390/polym12102262.
- Dorishetty, P.; Dutta, N.K.; Choudhury, N.R. Bioprintable tough hydrogels for tissue engineering applications. Adv. Colloid Interface Sci. 2020, 281, 102163, doi:10.1016/j.cis.2020.102163. .
- Compaan, A.M.; Christensen, K.; Huang, Y. Inkjet Bioprinting of 3D Silk Fibroin Cellular Constructs Using Sacrificial Alginate. ACS Biomater. Sci. Eng. 2017, 3, 1519–1526, doi:10.1021/acsbiomaterials.6b00432. .
- Jose, R.R.; Rodriguez, M.J.; Dixon, T.A.; Omenetto, F.; Kaplan, D.L. Evolution of Bioinks and Additive Manufacturing Technologies for 3D Bioprinting. ACS Biomater. Sci. Eng. 2016, 2, 1662–1678, doi:10.1021/acsbiomaterials.6b00088.
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. B Rev. 2008, 14, 149–165, doi:10.1089/ten.teb.2007.0332.
- Wust, S.; Godla, M.E.; Muller, R.; Hofmann, S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014, 10, 630–640, doi:10.1016/j.actbio.2013.10.016.
- Duffy, R.M.S.Y.; Feinberg, A.W. Understanding the role of ECM protein composition and geometric micropatterning for engineering human skeletal muscle. Ann. Biomed. Eng. 2016, 44, 2076–2089, doi:10.1007/s10439-016-1592-8.
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758, doi:10.1126/sciadv.1500758.
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 2014, 6, 035020, doi:10.1088/1758-5082/6/3/035020.
- Duarte Campos, D.F.; Blaeser, A.; Weber, M.; Jakel, J.; Neuss, S.; Jahnen-Dechent, W.; Fischer, H. Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid. Biofabrication 2013, 5, 015003, doi:10.1088/1758-5082/5/1/015003.
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng. C Methods 2011, 17, 79–87, doi:10.1089/ten.tec.2010.0359.
- Yan, D.; Zeng, B.; Han, Y.; Dai, H.; Liu, J.; Sun, Y.; Li, F. Preparation and laser powder bed fusion of composite microspheres consisting of poly(lactic acid) and nano-hydroxyapatite, Addit. Manuf. 2020, 34, 101305, doi.org/10.1016/j.addma.2020.101305.
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, and challenges. Prog. Mater. Sci. 2020, 100707, doi:10.1016/j.pmatsci.2020.100707.
- Gonzalez, J.; Mireles, J.; Lin, Y.; Wicker, R. Characterization of ceramic components fabricated using binder jetting additive manufacturing technology. Ceram. Int. 2016, 42, 10559–10564, doi:10.1016/j.ceramint.2016.03.079.
- Pu'ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Review on the fabrication of fused deposition modelling (FDM) composite filament for biomedical applications. Mater. Today Proc. 2020, 29, 228–232, doi:10.1016/j.matpr.2020.05.535.
- Yap, Y.L.; Wang, C.; Sing, S.L.; Dikshit, V.; Yeong, W.Y.; Wei, J. Material jetting additive manufacturing: An experimental study using designed metrological benchmarks. Precis. Eng. 2017, 50, 275–285, doi:10.1016/j.precisioneng.2017.05.015.
- Davoudinejad, A.; Diaz-Perez, L.C.; Quagliotti, D.; Pedersen, D.B.; Albajez-García, J.A.; Yagüe-Fabra, J.A.; Tosello, G. Additive manufacturing with vat polymerization method for precision polymer micro components production. Procedia CIRP 2018, 75, 98–102, doi:10.1016/j.procir.2018.04.049.
- Woesz, A.; Rumpler, M.; Stampfl, J.; Varga, F.; Fratzl-Zelman, N.; Roschger, P.; Klaushofer, K.; Fratzl, P. Towards bone replacement materials from calcium phosphates via rapid prototyping and ceramic gelcasting. Mater. Sci. Eng. C 2005, 25, 181–186, doi:10.1016/j.msec.2005.01.014.
- Lakhdar, Y.; Tuck, C.; Binner, J.; Terry, A.; Goodridge, R. Additive manufacturing of advanced ceramic materials. Prog. Mater. Sci. 2021, 116, 100736, doi:10.1016/j.pmatsci.2020.100736.
- Shuai, C.; Gao, C.; Nie, Y.; Hu, H.; Zhou, Y.; Peng, S. Structure and properties of nano-hydroxypatite scaffolds for bone tissue engineering with a selective laser sintering system. Nanotechnology 2011, 22, 285703, doi:10.1088/0957-4484/22/28/285703.
- Miranda, P.; Pajares, A.; Saiz, E.; Tomsia, A.P.; Guiberteau, F. Fracture modes under uniaxial compression in hydroxyapatite scaffolds fabricated by robocasting. J. Biomed. Mater. Res. A 2007, 83, 646–655, doi:10.1002/jbm.a.31272.
- Gao, Y.; Cao, W.L.; Wang, X.Y.; Gong, Y.D.; Tian, J.M.; Zhao, N.M.; Zhang, X.F. Characterization and osteoblast-like cell compatibility of porous scaffolds: Bovine hydroxyapatite and novel hydroxyapatite artificial bone. J. Mater. Sci. Mater. Med. 2006, 17, 815–823, doi:10.1007/s10856-006-9840-3.
- Warnke, P.H.; Seitz, H.; Warnke, F.; Becker, S.T.; Sivananthan, S.; Sherry, E.; Liu, Q.; Wiltfang, J.; Douglas, T. Ceramic scaffolds produced by computer-assisted 3D printing and sintering: Characterization and biocompatibility investigations. J. Biomed. Mater. Res. B 2010, 93, 212–217, doi:10.1002/jbm.b.31577.
- Gmeiner, R.; Deisinger, U.; Schonherr, J.; Lechner, B.; Detsch, R.; Boccaccini, A.; Stampfl, J. Additive manufacturing of bioactive glasses and silicate bioceramics. J. Ceram. Sci. Technol. 2015, 6, 75–86, doi:10.4416/JCST2015-00001.
- Shuai, C.; Mao, Z.; Han, Z.; Peng, S.; Li, Z. Fabrication and characterization of calcium silicate scaffolds for tissue engineering. J. Mech. Med. Biol. 2014, 14, 1450049, doi:10.1142/S0219519414500493.
- Vorndran, E.; Klarner, M.; Klammert, U.; Grover, L.M.; Patel, S.; Barralet, J.E.; Gbureck, U. 3D Powder Printing of Beta-Tricalcium Phosphate Ceramics Using Different Strategies. Adv. Eng. Mater. 2008, 10, B67–B71, doi:10.1002/adem.200800179.
- Felzmann, R.; Gruber, S.; Mitteramskogler, G.; Tesavibul, P.; Boccaccini, A.R.; Liska, R.; Stampfl, J. Lithography-Based AdditiveManufacturing of Cellular Ceramic Structures. Adv. Eng. Mater. 2012, 14, 1052–1058, doi:10.1002/adem.201200010.
- Bian,W.; Li, D.; Lian, Q.; Zhang,W.; Zhu, L.; Li, X.; Jin, Z. Design and fabrication of a novel porous implant with pre-set channels based on ceramic stereolithography for vascular implantation. Biofabrication 2011, 3, 034103, doi:10.1088/1758-5082/3/3/034103.
- Bose, S.; Darsell, J.; Kintner, M.; Hosick, H.; Bandyopadhyay, A. Pore size and pore volume effects on alumina and TCP ceramic scaffolds. Mater. Sci. Eng. C. 2003, 23, 479–486, doi:10.1016/S0928-4931(02)00129-7.
- Butscher, A.; Bohner, M.; Roth, C.; Ernstberger, A.; Heuberger, R.; Doebelin, N.; von Rohr, P.R.; Müller, R. Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds. Acta Biomater. 2012, 8, 373–385, doi:10.1016/j.actbio.2011.08.027.
- Partee, B.; Hollister, S.J.; Das, S. Selective Laser Sintering Process Optimization for Layered Manufacturing of CAPAR 6501 Polycaprolactone Bone Tissue Engineering Scaffolds. J. Manuf. Sci. Eng. 2005, 128, 531–540, doi:10.1115/1.2162589.
- Chen, C.H.; Lee, M.Y.; Shyu, V.B.H.; Chen, Y.C.; Chen, C.T.; Chen, J.P. Surface modification of polycaprolactone scaffolds fabricated via selective laser sintering for cartilage tissue engineering. Mater. Sci. Eng. C 2014, 40, 389–397, doi:10.1016/j.msec.2014.04.029.
- Shor, L.; Güçeri, S.; Chang, R.; Gordon, J.; Kang, Q.; Hartsock, L.; An, Y.; Sun, W. Precision extruding deposition (PED) fabrication of polycaprolactone (PCL) scaffolds for bone tissue engineering. Biofabrication 2009, 1, 015003, doi:10.1088/1758-5082/1/1/015003.
- Sobral, J.M.; Caridade, S.G.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. 2011, 7, 1009–1018, doi:10.1016/j.actbio.2010.11.003.
- Korpela, J.; Kokkari, A.; Korhonen, H.; Malin, M.; Närhi, T.; Seppälä, J. Biodegradable and bioactive porous scaffold structures prepared using fused deposition modeling. J. Biomed. Mater. Res. B 2013, 101, 610–619, doi:10.1002/jbm.b.32863.
- Ge, Z.; Wang, L.; Heng, B.C.; Tian, X.F.; Lu, K.; Tai Weng Fan, V.; Yeo, J.F.; Cao, T.; Tan, E. Proliferation and Differentiation of Human Osteoblasts within 3D printed Poly-Lactic-co-Glycolic Acid Scaffolds. J. Biomater. Appl. 2009, 23, 533–547, doi:10.1177/0885328208094301.
- Lee, M.; Wu, B.M.; Dunn, J.C.Y. Effect of scaffold architecture and pore size on smooth muscle cell growth. J. Biomed. Mater. Res. A 2008, 87, 1010–1016, doi:10.1002/jbm.a.31816.
- Lee, M.; Dunn, J.C.Y.;Wu, B.M. Scaffold fabrication by indirect three-dimensional printing. Biomaterials 2005, 26, 4281–4289, doi:10.1016/j.biomaterials.2004.10.040.
- Guo, T.; Holzberg, T.R.; Lim, C.G.; Gao, F.; Gargava, A.; Trachtenberg, J.E.; Mikos, A.G.; Fisher, J.P. 3D printing PLGA: A quantitative examination of the effects of polymer composition and printing parameters on print resolution. Biofabrication 2017, 9, 024101, doi:10.1088/1758-5090/aa6370.