4. Key Factors in Biobeds’ Effectiveness
4.1. Biomixture
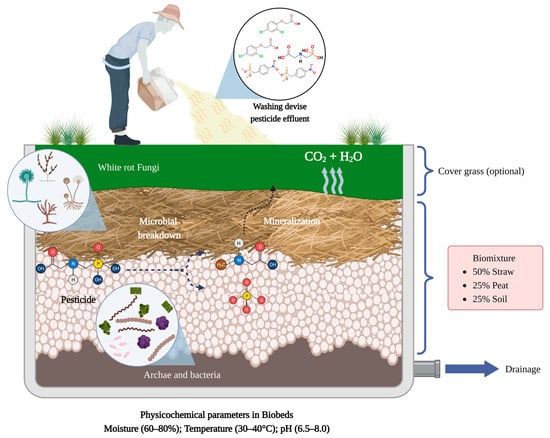
The composition of the biomixture is a key factor for the efficiency of pesticide degradation in biobed systems; so, each component (wheat straw, soil, and peat) plays an important role
[62]. For example, wheat straw is a lignocellulosic substrate that acts as an adsorbent for pesticides in the system, serves as physical support for the development of microbial communities, provides essential nutrients for the growth of fungi and bacteria, and stimulates the production of ligninolytic enzymes, such as laccases and peroxidases, reported to be highly efficient in the degradation of different pesticides. The soil supplies microorganisms to the system and stimulates the microbial activity that mediates the degradation of the pesticides, while peat is a porous material that increases pesticide retention in the biobed system, regulates the moisture, and reduces the pH, factors that favor pesticide dissipation
[63][64][65][66][63,64,65,66].
In the biomixture, wheat straw can be replaced by other lignocellulosic substrates, depending on the availability of these materials in a particular country where biobed systems are applied. For example, Karanasios et al. (2010)
[67] reported the use of different low-cost lignocellulosic materials, such as sunflower crop residues, olive leaves, grape stalks, orange peels, corn cobs, and spent mushroom substrate for the degradation of mixtures of pesticides in biobed systems. In this study, the alternative substrates favored the retention of pesticides in the system, and comparable pesticide half-life values, concerning those observed in the biobeds with the presence of wheat straw, were documented.
In another study, Diez et al. (2013)
[68] complemented the biomixture composition with the addition of lignocellulosic materials, such as pine sawdust (25%) and barley husk (25%), for the degradation of the pesticides carbendazim, isoproturon, and chlorpyrifos. The systems that contained wheat straw/barley husk (25%/25%) showed higher degradation percentages for carbendazim and chlorpyrifos after 90 days compared to the systems with only wheat straw (50%) and wheat straw/pine sawdust (25%/25%).
In a similar study, Urrutia et al. (2013)
[66] evaluated the addition of lignocellulosic materials such as barley husk, oat husk, and sawdust to biobed biomixtures for the treatment of the pesticides atrazine, chlorpyrifos, and isoproturon. Among the three lignocellulosic materials, oat husk was the best substitute for wheat straw, with similar pesticide degradation rates compared to the biomixture that included just wheat straw. In contrast, barley husk and sawdust can be added to the biomixtures in combination with wheat straw but not as the sole lignocellulosic material in the biomixture composition.
Gongora-Echeverría et al. (2017)
[69] evaluated the suitability of wheat straw substitution in biobed systems, employing different materials of high availability in southeastern Mexico such as compost, sisal fibers, corn stoves, and seaweed in combination with soil for the treatment of a pesticide mixture composed of 2,4-dichloro phenoxy acetic acid (2,4-D, 1.08 mg/cm
3 of mixture), atrazine (2.5 mg/cm
3 of mixture), carbofuran (0.23 mg/cm
3 of mixture), diazinon (0.34 mg/cm
3 of mixture), and glyphosate (0.36 mg/cm
3 of mixture), mimicking the composition of pesticide effluents generated by farmers in Yucatan, Mexico. In all evaluated biomixtures, the five pesticides’ dissipation was over 99% after 41 days.
Peat is an important component in biobed biomixtures; however, in some regions, this material has low availability or high cost. So, in biomixtures, peat has been substituted by alternative material or just eliminated from the biomixture composition
[70][71][70,71]. Among the alternative materials to peat for biobed mixtures, compost
[72][73][74][75][76][72,73,74,75,76] or vermicompost
[43][77][78][79][43,77,78,79] are notable for being the most reported.
Various agro-industrial wastes have been employed in the biomixture composition in biobed systems to treat fungicides, herbicides, and insecticides from different chemical families. They include spent coffee grounds
[80], coir
[80], cotton crop residues
[81][82][81,82], garden wastes
[83][84][83,84], livestock manure
[80][85][80,85], olive leaves
[68][86][87][68,86,87], pine bark
[80], and sewage sludge
[88]. Spent mushroom substrates and biochar have also been incorporated into the biomixture composition as complementary materials in pesticide dissipation
[38][39][42][70][89][90][91][38,39,42,70,89,90,91].
4.2. Microorganisms
In the biobed systems, the microbiota colonizing the biomixture are responsible for the pesticide degradation. Microorganisms such as bacteria and fungi may use pesticide molecules such as carbon, nitrogen, phosphorous, and energy sources for their growth. The efficient pesticide degradation by microorganisms is related to their great genetic plasticity, the production of diverse pesticide-degrading enzymes, fast growth, and adaptability to living in polluted environments
[89].
The materials that integrate the biomixture retain the pesticide molecules in the biobeds and serve as a habitat for the development of different soil autochthonous microorganisms
[92]. In biobeds, the presence of lignocellulosic materials reduces the pH in the system generating an environment that favors the growth and development of lignin-degrading fungi, such as different species of white-rot fungi
[45]. White-rot fungi are organisms broadly reported in the biodegradation of several organic pollutants, including pesticides from different chemical families
[93][94][95][96][97][98][93,94,95,96,97,98]. Fungi can produce extracellular enzymes, such as peroxidases, laccases, and the cytochrome P450 complex, implicated in pesticide degradation
[99][100][101][99,100,101]. On the other hand, the presence of peat in the biomixture also favors the development of white-rot fungi in biobeds; however, in biomixtures without peat, the pesticide degradation is mediated mainly by the bacterial community
[45]. Bacteria may act in synergy with fungi to enhance the pesticide and derived metabolites degradation; so, they can also produce different pesticide-degrading enzymes, such as dehalogenases, hydrolases, oxidoreductases, oxygenases, and esterases
[102][103][104][105][106][102,103,104,105,106].
In biobed systems, the biomixture supports the development of broad microbial diversity, and recent studies have evaluated such microbial complexity. For example, through a metagenomics approach, Bergsveinson et al. (2018)
[107] assessed the bacterial and fungal diversity in four biobed systems employed for treating pesticide rinsates with differential composition and pesticide concentrations. As a result of the study, around 440 bacterial genera and an average of 285 fungal genera were identified in each biobed system. In a similar study, Góngora-Echeverría et al. (2018)
[108] identified several archaea (23), bacteria (598), and fungi (64) species in lab-scale biobed systems with the presence of a mixture of commercial pesticide formulates (2,4-D, atrazine, carbofuran, diazinon, and glyphosate). In addition, Russell et al. (2021)
[109] evaluated the bacterial diversity in a two-cell biobed system. After the treatment of pesticide residues, in cell one, 81 bacterial species from 58 genera were identified, while in cell two, 36 bacterial species from 33 genera were identified. The most representative bacterial genera in both biobed cells were
Afipia,
Sphingopyxis, and
Pseudomonas.
The development of a great diversity of microorganisms in the biobed systems is crucial for the efficient treatment of pesticide residues. However, the metabolic activities of the indigenous microbiota do not always guarantee total pesticide degradation. Due to this, bioaugmentation strategies have been employed to enhance pesticide biodegradation efficiency in biobed systems. This strategy is based on the addition of selected endogenous or exogenous microorganisms, such as specific fungi and bacterial strains, or the use of characterized or non-characterized microbial consortia
[45][52][45,52]. The key characteristics for selecting microorganisms for a bioaugmentation strategy include pesticide resistance, high pesticide degradation efficiency, fast growth, and simple culture in lab conditions
[110][111][110,111]. Examples of microorganisms used in biobed bioaugmentation strategies include uncharacterized microbial consortia, archaea species, bacteria of different phyla such as
Actinobacteria (
Streptomyces spp.),
Bacteroidetes,
Firmicutes, and mainly Proteobacteria (
Achromobacter ssp.,
Bordetella ssp.,
Pseudomonas ssp., and
Variovorax ssp.), and white-rot fungi from different classes (
Aphelidiomycetes and
Pezizomycetes) and species (
Trametes versicolor and
Stereum hirsutum).
4.3. Physicochemical Parameters
Pesticide dissipation effectiveness in biobed systems is strongly related to the biomixture composition and the metabolic activity of the different microorganisms; however, other key parameters include the pre-incubation time, moisture, temperature, and pesticide concentration in the system. Fernández-Alberti et al. (2012)
[112] evaluated the effect of the biomixture pre-incubation time and moisture on chlorpyrifos (insecticide) degradation. In the study, the biomixture was composed of wheat straw, peat, and soil (2:1:1), pre-incubation took place (25 ± 1 °C) over 0, 15, and 30 days, and three water-holding-capacity percentages (WHC 40%, 60%, and 80%) were evaluated. The best condition for chlorpyrifos degradation (>70%) was 15 days pre-incubation and 60% WHC. Pre-incubation favors the microbial community proliferation in the biomixture, while at a high moisture (60% WHC), the ligninolytic enzyme activity in the biomixture increases.
In a similar study, Tortella et al. (2012)
[113] evaluated the effect of the biomixture maturity and concentration on the chlorpyrifos degradation; the biomixture (wheat straw, peat and soil, 2:1:1) was pre-incubated over 0, 15, and 30 days; after that time, three chlorpyrifos concentrations (200, 320, and 480 mg·kg
−1) were added to the biomixture. The biomixture’s maturity did not affect the chlorpyrifos degradation; all the biomixtures showed degradation percentages above 50%. However, increasing the chlorpyrifos concentration reduced the degradation efficiency and the hydrolytic and phenoloxidase activities in the systems. More recently, Kumari et al. (2019)
[114] evaluated the effect of pre-incubation, pesticide concentration, and moisture on the degradation process of azoxystrobin (fungicide) and imidacloprid (insecticide) in biobed systems, employing biomixtures that included rice straw/corn cobs, peat, and compost (2:1:1). Ten days of biomixture pre-incubation before pesticide application reduced by 5–9 times the degradation rate of the insecticide imidacloprid, while the increase in the WHC from 60% to 80% had a positive effect on the degradation rates of both pesticides, reducing their half-life time. However, increases in the concentration of the pesticides from 30 to 100 mg·kg
−1 reduced the degradation rates of both pesticides.
Cordova-Méndez et al. (2021)
[46] evaluated the effect of moisture and temperature on the dissipation of five pesticides, two insecticides: carbofuran and diazinon, and three herbicides: atrazine, 2,4-D, and glyphosate; five temperatures (5, 15, 25, 35, and 45 °C) and five water holding capacity percentages (20%, 40%, 60%, 80%, and 100%) were evaluated. The increasing temperature positively affected the dissipation of the five pesticides evaluated; the highest dissipation percentages were observed at temperatures of 35 and 45 °C. However, the increase in the water-holding percentages did not show a significant improvement in pesticide dissipation. The observed increase in pesticide dissipation was related to higher microbial activity at higher temperatures. According to the reviewed studies, for efficient pesticide treatment in biobed systems, physicochemical parameters, such as the preincubation time, moisture, temperature, and pesticide concentration in the system, must be optimized.
4.4. Analysis of the Biobeds’ Treated Effluents
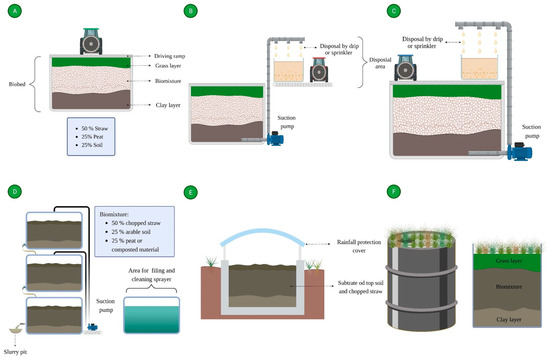
According to the biobed design, the systems are isolated from the soil through an impermeable layer, and it has been proposed that treated effluents ending from biobed systems could be reused for crop irrigation
[115]. However, leachate analysis in biobed systems is essential to guarantee the efficiency of the treatment process and avoid releasing pesticides into the environment, causing soil and water pollution. In this sense, Henriksen et al. (2003)
[58] evaluated the dissipation of the herbicides mecoprop and isoproturon in a biobed system. To determine the pesticide dissipation, the concentration of both herbicides in the leachate was assessed after a year, the concentrations of isoproturon and mecoprop were of 1.4% and 13%, respectively, of the initial dose (8 g), and the presence of a higher concentration of mecoprop in the biobed leachate was associated with its lower retention in the biobed biomixture.
The excessive effluent load in the biobed systems could be a limiting factor for pesticide retention and dissipation efficiency, causing the release of leachates with pesticide concentration above the limits established in the regulations. Foog et al. (2004)
[59] evaluated the effect of reducing the effluent loads in a biobed system (1.5 m deep) over the concentration of pesticides in leachates. They observed that a reduction from 1175 to 688 L/m
2 of biomixture decreased the pesticide concentration in leachate from <0.32% to <0.006%, while a decrease to 202 L/m
2 reduced the pesticide concentration to <0.0001%; according to their results, the maximum water holding in the system for efficient pesticide dissipation was 1121 L/m
2. In another study, Spliid et al. (2006)
[116] evaluated the presence of pesticides in leachates from a biobed system. The concentrations of 21 pesticides (5 g each in the mixture) were assessed through LC-MS/MS; after 593 days of treatment, only the herbicide bentazone showed a significant presence in leachates (14% of the original dose), ten pesticides were not detected in leachates, and the ten remaining pesticides showed reductions below 2% of the initial dose. The authors concluded that the biobeds were effective in retaining and degrading pesticides, generating effluents with lower pesticide concentrations.
More recently, Karas et al. (2015)
[38] evaluated the risk associated with the environmental release of the biobed-depurated wastewater, the leachates containing fungicides from pilot biobed systems. The leachates included traces of fungicides (diphenylamine, imazalil, ortho-phenylphenol, and thiabendazole); acute effects were evaluated in aquatic organisms, such as the crustacean
Daphnia magna and the fish
Oncorhynchus mykiss, while chronic effects were assessed in the fish
Oncorhynchus mykiss, the algae
Pseudokirchneriella subcapitata, and sediment-dwelling invertebrates such as
Chironomus sp. The biobed-depurated effluents with diphenylamine, imazalil, and ortho-phenylphenol did not show either an acute or chronic exposure risk in any bioindicator organism; only the effluents with thiabendazole showed an acute exposure risk for
Daphnia magna and a chronic exposure risk for
Oncorhynchus mykiss. In the same study, the treatment of fungicides using bioaugmentation with fungicide-degrader bacteria generated effluents that showed no acute or chronic exposure risk for the organisms evaluated. The authors conclude that biobed-treated effluents do not represent an environmental risk and can be safely disposed.