Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Carla Giordano and Version 2 by Peter Tang.
The aorta is the largest elastic artery in the human body and is classically divided into two anatomical segments, the thoracic and the abdominal aorta, separated by the diaphragm. The thoracic aorta includes the aortic root, the ascending aorta, the arch, and the descending aorta. The aorta’s elastic properties depend on its wall structure, composed of three distinct histologic layers: intima, media, and adventitia. The different aortic segments show different embryological and anatomical features, which account for their different physiological properties and impact the occurrence and natural history of congenital and acquired diseases that develop herein.
- thoracic aorta
- embryology
- aortic aneurysm
- aortic dissection
1. Introduction
The aorta is the largest elastic artery in the human body and is classically divided into two anatomical segments, the thoracic aorta (TA) and the abdominal aorta (AA), separated by the diaphragm. The different segments show distinctive physiological properties reflecting their different anatomical structure and embryology [1]. The TA presents higher compliance as compared to the AA and its elastic capacity, especially in the proximal segments, actively contributes to maintaining diastolic pressure and blood flow at the level of peripheral circulation.
The heterogeneous histogenesis of the different segments of the aorta impacts the onset and natural history of congenital and acquired diseases that develop herein. The latter may present as a chronic, often asymptomatic disorder (i.e., thoracic and abdominal aortic aneurisms, TAA and AAA) or as an acute life-threatening condition i.e., acute aortic syndromes (AAS).
2. Topographic Anatomy of the Thoracic Aorta
The thoracic aorta includes the aortic root, the ascending tract, the arch, and the descending aorta (Figure 1A–C) [2].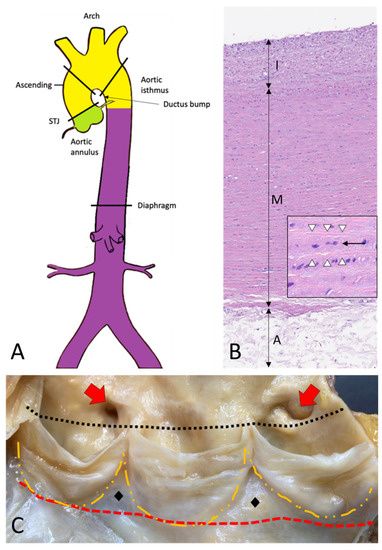
Figure 1. Topographic anatomy and normal histology of the aorta. (A)—Diagram of the aorta with different colors reflecting the different histogenesis of the SMCs of the tunica media: SMCs of the aortic root derive from the secondary heart field (lateral plate mesoderm) (green); SMCs of ascending aorta and the arch derive from the neural crests (ectoderm) (yellow); SMCs of the descending and abdominal aorta derive from the somites of the paraxial mesoderm (purple). SMCs = smooth muscle cells. (B)—Histological anatomy of the descending aorta with tunica intima (I), media (M), and adventitia (A). The mild fibrous intimal thickening is a typical age-related change. The normal lamellar architecture of the tunica media is preserved and showed at higher magnification in the insert, with its elastic laminae (white arrowheads) and the intervening smooth muscle cells (black arrow) (Hematoxylin and eosin, original magnification 10×; insert: 40×). (C)—The aortic root is the segment between the annulus (red dashed line) and the sinotubular junction (black dotted line) and comprises the sinuses of Valsalva with the aortic cusps (outlined with the orange dashed line), the origin of the coronary arteries (red arrows), and the intercuspal triangles (black diamonds).
3. Histology and Histogenesis of the Aorta
The aorta’s elastic properties depend on its wall structure, composed of three distinct histologic layers: intima, media, and adventitia (Figure 1B). The media is the largest component and is formed by concentrically organized lamellar units. Each lamellar unit is composed of two layers of elastic laminae and smooth muscle cells (SMCs), collagen fibers, and proteoglycans lining in between (Figure 1B, insert). The number and thickness of the lamellar units of the media increase with age and, in adults, vary according to topographic location [5]. During postnatal growth, the increase in medial thickness in the ascending aorta and arch is achieved through an increase in the number of lamellae (up to 60 in adulthood), whereas in the descending aorta through the thickening of the existing lamellar units (up to 28–30). This different behavior reflects the different embryological origins of SMCs at different topographic sites (Figure 1A), which in turn condition the response of SMCs to growth factor stimuli (e.g., TGF-β). The SMCs of the ascending aorta and the arch derive from the neural crests (i.e., from the ectoderm), while those of the descending aorta derive from the somites of the paraxial mesoderm [6][7][8][6,7,8]. Of note, the ratio between the aortic diameter and the medial thickness remains constant in the different segments of the aorta [9]. Thanks to the higher number of lamellar units, the TA is more compliant as compared to the AA. The aorta does not contain a distinct inner or outer elastic lamina. At birth, the intima is thin and consists of the endothelium alone, in close contact with the first elastic lamella. In the adult, the accumulation of extracellular matrix proteins and mesenchymal cells induces an increase in intimal thickness (Figure 1B). The adventitia comprises loose connective tissue, vasa vasorum, and lymphatic vessels. Vasa vasorum normally extends into the outer third of the media.4. Embryology of the Aorta
4.1. Embryology of the Aortic Root
The aortic root development is strictly connected with the embryology of the cardiac outflow tract (Figure 2). In midweek 5 of embryogenesis, cells from the neural crest migrate in the truncus arteriosus (the cranial portion of the primitive heart tube) and contribute to the formation of a spiral conotruncal septum, which separates the aortic and pulmonary outflow tracts [8]. From the wall of each tract, three endocardial swellings give rise to the cusps of the semilunar valves of the aorta and pulmonary artery. Excavation of truncal tissue inferior to the aortic swellings leads to the formation of the sinus of Valsalva.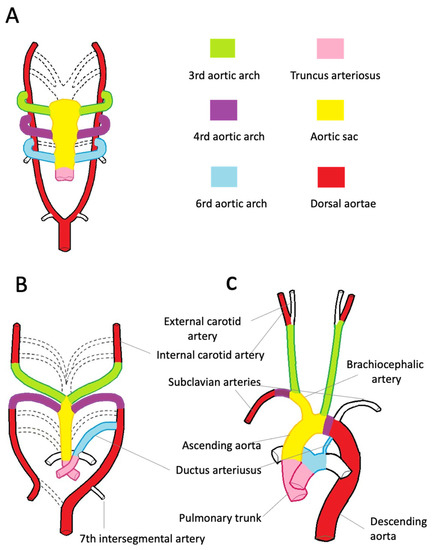
Figure 2. Embryologic development of the thoracic aorta and its branches. (A)—The scheme highlights the development of the six arches from the aortic sac during the 5th week of development, the 1st and 2nd arches have regressed (dashed line). The truncus arteriosus is partially divided by the conotruncal septum. (B)—Aortic arches at 7 weeks of development. The segments of the dorsal aorta connecting the third and fourth arch arteries disappear, as well as the fifth, part of the right and sixth arches and a portion of the right dorsal aorta. (C)—At 8 weeks, the thoracic aorta and the epiaortic vessels have almost completed their development. Note the patency of the ductus arteriosus, originating from the left sixth aortic arch.
