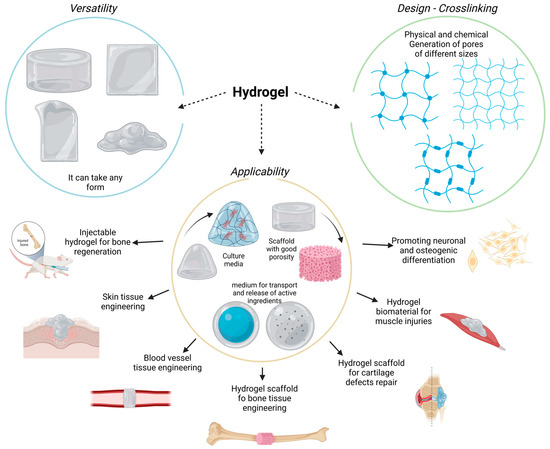
Hydrogels are versatile biomaterials characterized by three-dimensional, cross-linked, highly hydrated polymeric networks. These polymers exhibit a great variety of biochemical and biophysical properties, which allow for the diffusion of diverse molecules, such as drugs, active ingredients, growth factors, and nanoparticles. Meanwhile, these polymers can control chemical and molecular interactions at the cellular level. The polymeric network can be molded into different structures, imitating the structural characteristics of surrounding tissues and bone defects. Interestingly, the application of hydrogels in bone tissue engineering (BTE) has been gathering significant attention due to the beneficial bone improvement results that have been achieved.
- bone regeneration
- hydrogels
- polymers
- natural hydrogels
- bone tissue engineering
1. Introduction
2. Hydrogels
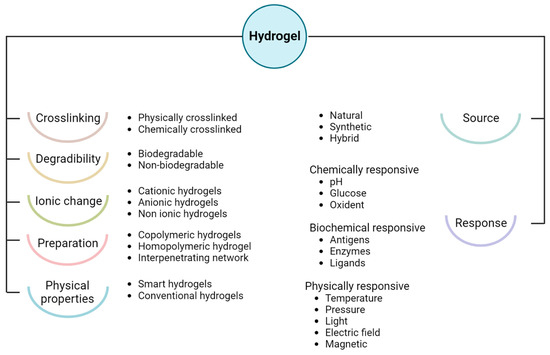
3. Hydrogel Classification
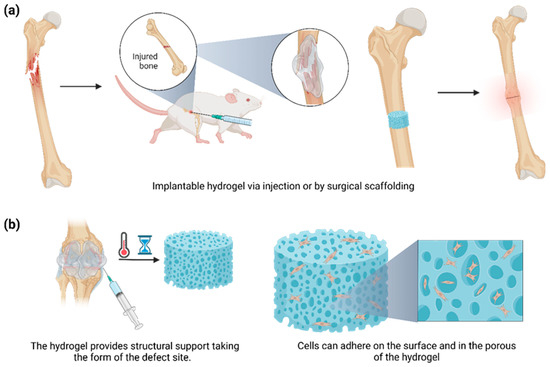
4. Hydrogels for Bone Regeneration
-
No cytotoxic and no immunogenic response, in order to avoid a chronic and non-regulable inflammatory reaction;
-
Osteoinductive, osteoconductive, osteogenic, and osteocompatible qualities for better bone anchorage and regeneration;
-
Mimicking the natural ECM at the implant site;
-
Degradable by different enzymes or environmental molecules, leaving sufficient space for new bone formation;
-
Resistant and stable during sterilization;
-
Controlling the size and interconnection of the pores to optimize the characteristics of drug release, cell growth, oxygen diffusion, and nutrient exchange;
-
Patient-friendly injectable form to reduce pain and simplify the administration process.
References
- Bates, P.; Yeo, A.; Ramachandran, M. Bone Injury, Healing and Grafting. In Basic Orthopaedic Sciences; CRC Press: Boca Raton, FL, USA, 2017; pp. 205–222.
- Einhorn, T.A. The Cell and Molecular Biology of Fracture Healing. Clin. Orthop. Relat. Res. 1998, 355S, S7–S21.
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66.
- Cui, W.; Liu, Q.; Yang, L.; Wang, K.; Sun, T.; Ji, Y.; Liu, L.; Yu, W.; Qu, Y.; Wang, J.; et al. Sustained Delivery of BMP-2-Related Peptide from the True Bone Ceramics/Hollow Mesoporous Silica Nanoparticles Scaffold for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 211–221.
- Mahanta, A.K.; Patel, D.K.; Maiti, P. Nanohybrid Scaffold of Chitosan and Functionalized Graphene Oxide for Controlled Drug Delivery and Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 5139–5149.
- Tzagiollari, A.; McCarthy, H.O.; Levingstone, T.J.; Dunne, N.J. Biodegradable and Biocompatible Adhesives for the Effective Stabilisation, Repair and Regeneration of Bone. Bioengineering 2022, 9, 250.
- Gupta, H.; Zioupos, P. Fracture of bone tissue: The ‘hows’ and the ‘whys’. Med. Eng. Phys. 2008, 30, 1209–1226.
- Nellans, K.W.; Kowalski, E.; Chung, K.C. The Epidemiology of Distal Radius Fractures. Hand Clin. 2012, 28, 113–125.
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592.
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Nanoengineered Osteoinductive and Elastomeric Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 590–600.
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. S3), S20–S27.
- Audigé, L.; Griffin, D.; Bhandari, M.; Kellam, J.; Rüedi, T.P. Path Analysis of Factors for Delayed Healing and Nonunion in 416 Operatively Treated Tibial Shaft Fractures. Clin. Orthop. Relat. Res. 2005, 438, 221–232.
- Petite, H.; Viateau, V.; Bensaïd, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963.
- Shrivats, A.R.; McDermott, M.C.; Hollinger, J.O. Bone tissue engineering: State of the union. Drug Discov. Today 2014, 19, 781–786.
- Chen, C.-Y.; Chen, C.-C.; Wang, C.Y.; Lee, A.K.-X.; Yeh, C.-L.; Lin, C.-P. Assessment of the Release of Vascular Endothelial Growth Factor from 3D-Printed Poly-ε-Caprolactone/Hydroxyapatite/Calcium Sulfate Scaffold with Enhanced Osteogenic Capacity. Polymers 2020, 12, 1455.
- Zyuzkov, G. Targeted Regulation of Intracellular Signal Transduction in Regeneration-Competent Cells: A new Direction for Therapy in Regenerative Medicine. Biointerface Res. Appl. Chem. 2021, 11, 12238–12251.
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237.
- Fibbe, W.E.; Dazzi, F.; LeBlanc, K. MSCs: Science and trials. Nat. Med. 2013, 19, 812–813.
- Ansari, M.; Eshghanmalek, M. Biomaterials for repair and regeneration of the cartilage tissue. Bio-Design Manuf. 2019, 2, 41–49.
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2021, 121, 10908–10949.
- Zhang, Y.; Yu, T.; Peng, L.; Sun, Q.; Wei, Y.; Han, B. Advancements in Hydrogel-Based Drug Sustained Release Systems for Bone Tissue Engineering. Front. Pharmacol. 2020, 11, 622.
- Janoušková, O. Synthetic Polymer Scaffolds for Soft Tissue Engineering. Physiol. Res. 2018, 67, S335–S348.
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408.
- Oliva, N.; Shin, M.; Burdick, J.A. Editorial: Special Issue on Advanced Biomedical Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 3993–3996.
- Zhang, Y.; Li, Z.; Guan, J.; Mao, Y.; Zhou, P. Hydrogel: A potential therapeutic material for bone tissue engineering. AIP Adv. 2021, 11, 010701.
- Ruedinger, F.; Lavrentieva, A.; Blume, C.; Pepelanova, I.; Scheper, T. Hydrogels for 3D mammalian cell culture: A starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 2015, 99, 623–636.
- Liao, H.T.; Tsai, M.-J.; Brahmayya, M.; Chen, J.-P. Bone Regeneration Using Adipose-Derived Stem Cells in Injectable Thermo-Gelling Hydrogel Scaffold Containing Platelet-Rich Plasma and Biphasic Calcium Phosphate. Int. J. Mol. Sci. 2018, 19, 2537.
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102.
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433.
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339.
- Short, A.R.; Koralla, D.; Deshmukh, A.; Wissel, B.; Stocker, B.; Calhoun, M.; Dean, D.; Winter, J.O. Hydrogels that allow and facilitate bone repair, remodeling, and regeneration. J. Mater. Chem. B 2015, 3, 7818–7830.
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417.
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339.
- Nabavi, M.H.; Salehi, M.; Ehterami, A.; Bastami, F.; Semyari, H.; Tehranchi, M.; Semyari, H. A collagen-based hydrogel containing tacrolimus for bone tissue engineering. Drug Deliv. Transl. Res. 2020, 10, 108–121.
- Salehi, M.; Naseri-Nosar, M.; Ebrahimi-Barough, S.; Nourani, M.; Vaez, A.; Farzamfar, S.; Ai, J. Regeneration of sciatic nerve crush injury by a hydroxyapatite nanoparticle-containing collagen type I hydrogel. J. Physiol. Sci. 2018, 68, 579–587.
- Gao, F.; Li, J.; Wang, L.; Zhang, D.; Zhang, J.; Guan, F.; Yao, M.-H. Dual-enzymatically crosslinked hyaluronic acid hydrogel as a long-time 3D stem cell culture system. Biomed. Mater. 2020, 15, 045013.
- Paez, J.I.; Farrukh, A.; Valbuena-Mendoza, R.; Włodarczyk-Biegun, M.K.; del Campo, A. Thiol-Methylsulfone-Based Hydrogels for 3D Cell Encapsulation. ACS Appl. Mater. Interfaces 2020, 12, 8062–8072.
- Silva, R.; Fabry, B.; Boccaccini, A.R. Fibrous protein-based hydrogels for cell encapsulation. Biomaterials 2014, 35, 6727–6738.
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544.
- Lee, S.-H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359.
- Guarino, V.; Caputo, T.; Altobelli, R.; Ambrosio, L. Degradation properties and metabolic activity of alginate and chitosan polyelectrolytes for drug delivery and tissue engineering applications. AIMS Mater. Sci. 2015, 2, 497–502.
