Mg-based materials, from a comprehensive consideration of energy storage performance, raw material reserves, and prices, have demonstrated potential industrial applications as large-scale hydrogen storage materials. Nevertheless, Mg-based materials also have obvious disadvantages: as a hydrogen storage material, the hydrogen absorption/desorption rate is insufficient, as well as the high hydrogen absorption/desorption temperatures; as the electrode material of Ni-MH batteries, the reactions of Mg with alkaline electrolyte and corrosion are the main problems for applications.
- energy storage
- magnesium-based materials
- hydrogen storage
- surface modification
- nickel–metal hydride batteries
1. Introduction
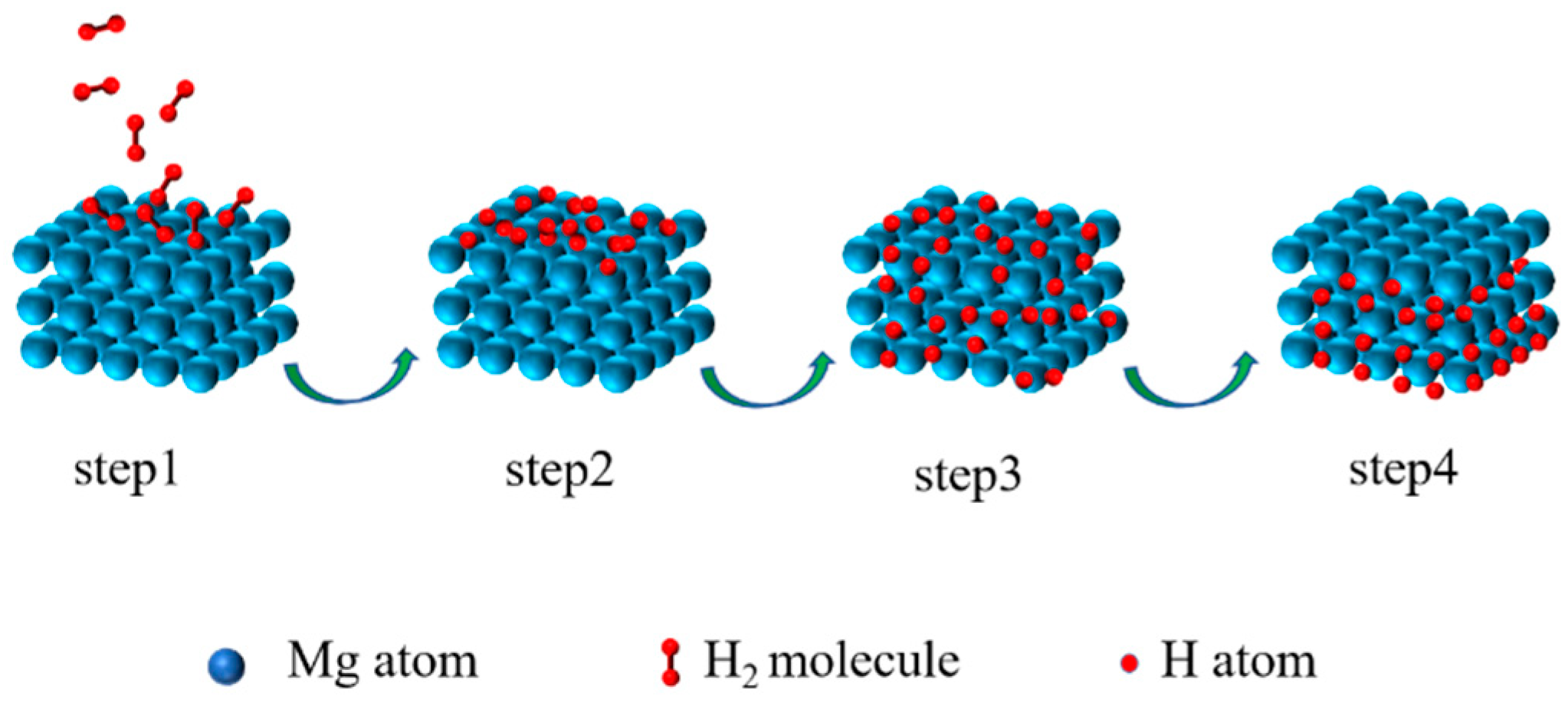
2. Surface Modification of Magnesium-Based Materials
2.1. Surface Modifications of Hydrogen Storage Material
2.2. Surface Modifications of Electrode Materials in Nickel–Metal Hydride Batteries
Ni-MH batteries are secondary new energy batteries with Ni(OH)2 as the positive electrode, metal hydride (MH) as the negative electrode, and an alkaline solution as the electrolyte [108,109][47][48]. Ni-MH batteries have been widely used in commercial hybrid vehicles, such as Toyota, Honda, and Ford, since 1999 due to their high level of safety, environmental friendliness, and good low-temperature properties [110,111][49][50]. However, the capacity, durability, and discharge performance (kinetics) of the battery depend primarily on the inherent properties of the electrode materials, especially for the preparation of active materials for the negative electrode [112,113][51][52]. There have been a series of studies on negative electrode materials for Ni-MH batteries, such as AB5, AB2, AB3, AB, A2B, and Mg-based materials [114,115,116,117,118][53][54][55][56][57]. However, with these materials for Ni-MH battery applications in the field of high power, there are still some problems, such as easy polarization, a poor discharge ability at a high rate, easy corrosion in alkaline electrolytes, and poor cycling stability. The performance of Ni-MH batteries depends not only on the bulk structure of the electrode but also on its surface state. Mg-based alloys are widely used as the negative electrode in Ni-MH batteries due to their exceptional electrochemical properties, cost-effectiveness, and abundant reserves of raw materials [108][47]. However, the appearance and growth of its MgO/Mg(OH)2 surface layer impede electron transport at the electrode/electrolyte interface and create a large discharge potential, resulting in a lower discharge capacity and a poorer kinetic performance of the electrode material [119][58]. Moreover, the interfacial reaction between the electrode material and the electrolyte can seriously affect the electrochemical performance of the batteries [120,121][59][60]. The optimization of the performance of Mg-based electrode materials and Ni-MH batteries can be achieved through surface treatment. Common methods of modification include surface coating, nanocrystallization and amorphization, and surface catalysis.2.3. Analysis of the Application of Magnesium-Based Materials in Existing Fields
The research and development of energy storage materials are crucial to the utilization and storage of energy. The ideal energy storage materials for a wide range of industrial and residential should consider factors such as the raw material and preparation costs, energy storage capacity, operating conditions, and service life. Mg has abundant reserves (about 2% [157][61]) and good hydrogen storage properties in the Earth’s crust. Currently, the research on the performance of Mg-based materials in hydrogen storage mainly focuses on solid-state hydrogen storage and Ni-MH batteries. Although researchers have shown that Mg-based materials show relatively excellent performance in hydrogen storage and electrochemical energy storage, which still not enough to achieve large-scale adoption. In accordance with what is described in this paper, Table 1 and Table 2 briefly summarize the relevant properties of some modified Mg-based materials in the field of hydrogen storage and Ni-MH batteries, respectively.| Materials | Surface Treatment | ΔHde (kJ mol−1) |
|---|
(mA h g | −1 | ) |
| Capacity | (wt%) |
|---|
CRR | C n/C1(%) |
| T | ab | (°C) | T | de |
|---|
ECD | (mA g | −1 ) |
| (°C) | Ref. |
|---|
| Ref. | |||||
| Nd0.7Mg0.3Ni3 | Coating layer (Ni) | 355 | 79.6 (C200) | 202.6 | [131][75] |
| Nd0.7Mg0.3Ni3 | Coating layer (Co) | 337 | 76.5 (C200) | 163.6 | [131][75] |
| La0.8Mg0.2Ni3.4Al0.1 | Coating layer (polyaniline) | 391.8 | 87.5 (C100) | 98.9 | [129][76] |
| Mg2Ni | Coating layer (rGO) | 594 | 60 (C50) | 225.9 | [132][77] |
| Mg2Ni | Coating layer (Ni) | 946 | 38 (C10) | 91.6 | [128][78] |
| Mg0.9Ti0.1Ni | - | 455 | 31.6 (C20) | - | [139][79] |
| Mg0.8Ti0.1Pd0.1Ni | Amorphous structure | 326.5 | 74.2 (C20) | 184 | [139][79] |
|
La9Ce1Mg80Ni5 (Ni + 3 wt% GR) |
Nanocrystalline and amorphous structures (ball milling 20 h) |
315.4 | 73 (C20) | - | [140][80] |
|
La9Ce1Mg80Ni5 (Ni + 3 wt% GR) |
Nanocrystalline and amorphous structures (ball milling 80 h) | 344.9 | 55 (C20) | - | [140][80] |
|
La0.88Mg0.12Ni2.95Mn0.10Co0.55Al0.10 |
Surface catalytic phase (Ni-Cu-P) |
361 | 84.8 (C200) | 378.9 | [133][81] |
|
La0.75Mg0.25Ni3.2Co0.2Al0.1 |
Surface catalytic phase (Cu-Pd) |
386.3 | 82.7 (C100) | 483.8 | [28] |
|
La1.7Mg1.3Ni9 |
Surface catalytic phase (Y2O3, milled 10 h) | 392.2 | 90 (C30) | - | [151][82] |
|
La1.7Mg1.3Ni9 |
Surface catalytic phase (Y2O3, milled 20 h) | 387 | 95 (C30) | - | [151][82] |
| Mg | - | 74.5 | 7.6 | - | 420 | [158][62] |
| Mg | Surface catalytic phase (Ni@rGO) | - | 6.0 | - | 300 | [61][63] |
| Mg | Surface catalytic phase (Co@CNTs) | - | 6.89 | - | 300 | [62][64] |
| Mg | Surface catalytic phase (Fe3O4@GS) | 60.62 | 6.20 | 290 | - | [64][65] |
| Mg | Surface catalytic phase (LiCoO2) | 48.5 | 5.5 | 250 | - | [159][66] |
| Mg2FeH6 | Nanoparticles (<20 nm) |
74 | 5.2 | - | 355 | [76][67] |
| La7Sm3Mg80Ni10 | Nanostructure | 74.23 | 3 | 300 | - | [74][68] |
| Mg-Ti | Nanoparticles (<32 nm) |
71.3 | 5.2 | - | 190 | [79][69] |
| Mg-Ti | Core–shell structure | - | 5 | - | 250 | [96][70] |
| Mg | Core–shell structure (Mg-Er) |
69.9 | 7.37 | - | - | [98][71] |
| Mg | Core–shell structure (Mg NCs-PMMA) |
- | 5.97 | 200 | - | [104][72] |
| Mg | Core–shell structure (Mg-NiF2) |
- | 3.85 | 200 | - | [99][73] |
| Mg | Core–shell structure (Mg@Co@V) | 74.83 | - | - | 323 | [103][74] |
| Materials | Surface Treatment |
MDC
|
Note: (1) ) MDC indicates the maximum discharge capacity, CRR indicates the capacity retention rate, and ECD indicates the exchange current density (Cn indicates the capacity after the nth cycle). (2)“-” indicates that no data exist.
References
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613.
- Li, K.; Bian, H.; Liu, C.; Zhang, D.; Yang, Y. Comparison of geothermal with solar and wind power generation systems. Renew. Sustain. Energy Rev. 2015, 42, 1464–1474.
- Khojasteh, D.; Khojasteh, D.; Kamali, R.; Beyene, A.; Iglesias, G. Assessment of renewable energy resources in Iran; with a focus on wave and tidal energy. Renew. Sustain. Energy Rev. 2018, 81, 2992–3005.
- Hussain, A.; Arif, S.M.; Aslam, M. Emerging renewable and sustainable energy technologies: State of the art. Renew. Sustain. Energy Rev. 2017, 71, 12–28.
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327.
- Rosen, M.A.; Koohi-Fayegh, S. The prospects for hydrogen as an energy carrier: An overview of hydrogen energy and hydrogen energy systems. Energy Ecol. Environ. 2016, 1, 10–29.
- Pearson, P.J.G.; Foxon, T.J. A low carbon industrial revolution? Insights and challenges from past technological and economic transformations. Energy Policy 2012, 50, 117–127.
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676.
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552.
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62.
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems–Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250.
- Mahlia, T.M.I.; Saktisahdan, T.J.; Jannifar, A.; Hasan, M.H.; Matseelar, H.S.C. A review of available methods and development on energy storage; technology update. Renew. Sustain. Energy Rev. 2014, 33, 532–545.
- Song, Z.; Zhan, H.; Zhou, Y. Polyimides: Promising energy-storage materials. Angew. Chem. Int. Ed. 2010, 49, 8444–8448.
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.S.; Wang, H. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Storage Mater. 2019, 16, 545–573.
- Li, J.; Li, B.; Shao, H.; Li, W.; Lin, H. Catalysis and downsizing in Mg-Based hydrogen storage materials. Catalysts 2018, 8, 89.
- Li, B.; Li, J.; Shao, H.; He, L. Mg-based hydrogen absorbing materials for thermal energy storage—A Review. Appl. Sci. 2018, 8, 1375.
- Shao, H.; Xin, G.; Zheng, J.; Li, X.; Akiba, E. Nanotechnology in Mg-based materials for hydrogen storage. Nano Energy 2012, 1, 590–601.
- Zaluska, A.; Zaluski, L.; Ström–Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloy. Compd. 1999, 288, 217–225.
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Structural study and hydrogen sorption kinetics of ball-milled magnesium hydride. J. Alloy. Compd. 1999, 293, 495–500.
- Jerkiewicz, G. Hydrogen sorption ATIN electrodes. Prog. Surf. Sci. 1998, 57, 137–186.
- Züttel, A.; Güther, V.; Otto, A.; Bartsch, M.; Kotz, R.; Chartouni, D.; Nutzenadel, C.; Schlapbach, L. About the mechanism and the rate limiting step of the metalhydride electrode reaction. J. Alloy. Compd. 1999, 293–295, 663–669.
- Tliha, M.; Mathlouthi, H.; Lamloumi, J.; Percheron-Guegan, A. AB5-type hydrogen storage alloy used as anodic materials in Ni-MH batteries. J. Alloy. Compd. 2007, 436, 221–225.
- Cui, N.; Luo, J.L.; Chuang, K.T. Nickel–metal hydride (Ni-MH) battery using Mg2Ni-type hydrogen storage alloy. J. Alloy. Compd. 2000, 302, 218–226.
- Xiong, W.; Yan, H.; Wang, L.; Verbetsky, V.; Zhao, X.; Mitrokhin, S.; Li, B.; Li, J.; Wang, Y. Characteristics of A2B7-type LaYNi-based hydrogen storage alloys modified by partially substituting Ni with Mn. Int. J. Hydrogen Energy 2017, 42, 10131–10141.
- Wang, C.; Soriaga, M.P.; Srinivasan, S. Determination of reaction resistances for metal-hydride electrodes during anodic polarization. J. Power Sources 2000, 85, 212–223.
- Zhang, H.; Zheng, X.; Tian, X.; Liu, Y.; Li, X. New approaches for rare earth-magnesium based hydrogen storage alloys. Prog. Nat. Sci. Mater. Int. 2017, 27, 50–57.
- Wei, T.Y.; Lim, K.L.; Tseng, Y.S.; Chan, S.L.I. A review on the characterization of hydrogen in hydrogen storage materials. Renew. Sustain. Energy Rev. 2017, 79, 1122–1133.
- Wang, Y.; Tang, W.; Wang, F.; Ding, C.; Xu, S.; Yu, R. Effects of surface coating with Cu–Pd on electrochemical properties of A2B7-type hydrogen storage alloy. Int. J. Hydrogen Energy 2018, 43, 3244–3252.
- Shen, W.; Han, S.; Li, Y.; Yang, J.; Xiao, K. Electrochemical performance of polyaniline electroless deposited La–Mg–Ni-based hydrogen storage alloys. Mater. Chem. Phys. 2012, 132, 852–857.
- House, S.D.; Vajo, J.J.; Ren, C.; Rockett, A.A.; Robertson, I.M. Effect of ball-milling duration and dehydrogenation on the morphology, microstructure and catalyst dispersion in Ni-catalyzed MgH2 hydrogen storage materials. Acta Mater. 2015, 86, 55–68.
- Ouyang, L.Z.; Cao, Z.J.; Li, L.L.; Wang, H.; Liu, J.W.; Min, D.; Chen, Y.W.; Xiao, F.M.; Tang, R.H.; Zhu, M. Enhanced high-rate discharge properties of La11.3Mg6.0Sm7.4Ni61.0 Co7.2Al7.1 with added graphene synthesized by plasma milling. Int. J. Hydrogen Energy 2014, 39, 12765–12772.
- Zhang, Z.; Jin, J.; Zhang, H.; Qi, X.; Bian, Y.; Zhao, H. First-principles calculation of hydrogen adsorption and diffusion on Mn-doped Mg2Ni (010) surfaces. Appl. Surf. Sci. 2017, 425, 148–155.
- Li, Q.; Lu, Y.; Luo, Q.; Yang, X.; Yang, Y.; Tan, J.; Dong, Z.; Dang, J.; Li, J.; Chen, Y.; et al. Thermodynamics and kinetics of hydriding and dehydriding reactions in Mg-based hydrogen storage materials. J. Magnes. Alloy. 2021, 9, 1922–1941.
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33.
- Banerjee, S.; Pillai, C.G.S.; Majumder, C. Dissociation and diffusion of hydrogen on the Mg (0001) surface: Catalytic effect of V and Ni double substitution. J. Phys. Chem. C 2009, 113, 10574–10579.
- Martino, P.; Chiesa, M.; Cristina Paganini, M.; Giamello, E. Coadsorption of NO and H2 at the surface of MgO monitored by EPR spectroscopy. Towards a site specific discrimination of polycrystalline oxide surfaces. Surf. Sci. 2003, 527, 80–88.
- Liu, Y.; Cao, Y.; Huang, L.; Gao, M.; Pan, H. Rare earth–Mg–Ni-based hydrogen storage alloys as negative electrode materials for Ni/MH batteries. J. Alloy. Compd. 2011, 509, 675–686.
- Feng, F.; Geng, M.; Northwood, D.O. Electrochemical behaviour of intermetallic-based metal hydrides used in Ni/metal hydride (MH) batteries: A review. Int. J. Hydrogen Energy 2001, 26, 725–734.
- Zhao, X.; Ding, Y.; Ma, L.; Wang, L.; Yang, M.; Shen, X. Electrochemical properties of MmNi3.8Co0.75Mn0.4Al0.2 hydrogen storage alloy modified with nanocrystalline nickel. Int. J. Hydrogen Energy 2008, 33, 6727–6733.
- Zhu, Y.; Yang, C.; Zhu, J.; Li, L. Structural and electrochemical hydrogen storage properties of Mg2Ni-based alloys. J. Alloy. Compd. 2011, 509, 5309–5314.
- Zhu, M.; Lu, Y.; Ouyang, L.; Wang, H. Thermodynamic tuning of Mg-based hydrogen storage alloys: A Review. Materials 2013, 6, 4654–4674.
- Luo, Q.; Li, J.; Li, B.; Liu, B.; Shao, H.; Li, Q. Kinetics in Mg-based hydrogen storage materials: Enhancement and mechanism. J. Magnes. Alloy. 2019, 7, 58–71.
- Arboleda, J.N.B.; Kasai, H.; Nobuhara, K.; Diño, W.A.; Nakanishi, H. Dissociation and Sticking of H2 on Mg(0001), Ti(0001) and La(0001) Surfaces. J. Phys. Soc. Jpn. 2004, 73, 745–748.
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663.
- Sakintuna, B.; Lamaridarkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140.
- Aguey-Zinsou, K.F.; Ares-Fernández, J.R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543.
- Wang, W.; Liu, X.; Zhang, L.; Zhang, S.; Guo, W.; Zhao, Y.; Zhang, H.; Li, Y.; Han, S. The electrochemical characteristics of AB4-type rare earth-Mg–Ni-based superlattice structure hydrogen storage alloys for nickel metal hydride battery. J. Magnes. Alloy. 2021, 9, 2039–2048.
- Ouyang, L.; Huang, J.; Wang, H.; Liu, J.; Zhu, M. Progress of hydrogen storage alloys for Ni-MH rechargeable power batteries in electric vehicles: A review. Mater. Chem. Phys. 2017, 200, 164–178.
- Liu, Y.; Pan, H.; Gao, M.; Wang, Q. Advanced hydrogen storage alloys for Ni/MH rechargeable batteries. J. Mater. Chem. 2011, 21, 4743–4755.
- Young, K.H.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608.
- Kleperis, J.; Wojcik, G.; Czerwinski, A.; Skowronski, J.; Kopczyk, M.; Beltowska-Brzezinska, M. Electrochemical behavior of metal hydrides. J. Solid State Electrochem. 2001, 5, 229–249.
- Cuevas, F.; Joubert, J.M.; Latroche, M.; Percheron-Guégan, A. Intermetallic compounds as negative electrodes of Ni/MH batteries. Appl. Phys. A Mater. Sci. Process. 2001, 72, 225–238.
- Sünbül, S.E.; Öztürk, S.; İçin, K. Structural and hydrogen storage properties of Mg60-Ni40 and Mg80-Ni20 alloys prepared by planar flow casting. J. Mater. Eng. Perform. 2020, 29, 6101–6107.
- Charbonnier, V.; Madern, N.; Monnier, J.; Zhang, J.; Paul-Boncour, V.; Latroche, M. Relationship between H2 sorption, electrochemical cycling and aqueous corrosion properties in A5Ni19 hydride-forming alloys (A = Gd, Sm). J. Power Sources 2018, 397, 280–287.
- Wang, C.C.; Zhou, Y.T.; Yang, C.C.; Jiang, Q. A strategy for designing new AB4.5-type hydrogen storage alloys with high capacity and long cycling life. J. Power Sources 2018, 398, 42–48.
- Zhao, X.; Ma, L. Recent progress in hydrogen storage alloys for nickel/metal hydride secondary batteries. Int. J. Hydrogen Energy 2009, 34, 4788–4796.
- Jia, Z.; Zhang, L.; Zhao, Y.; Cao, J.; Li, Y.; Dong, Z.; Wang, W.; Han, S. Self-discharge characteristic and mechanism of single-phase PuNi3-, Gd2Co7-, and Pr5Co19-type Nd–Mg–Ni-based alloys. J. Power Sources 2017, 371, 188–196.
- Cui, N.; Luan, B.; Liu, H.K.; Dou, S.X. Discharge behaviour of Mg2Ni-typehydrogen-storage alloy electrodes in 6 M KOH solution by electrochemical impedance spectroscopy. J. Power Sources 1996, 63, 209–214.
- Li, M.M.; Yang, C.C.; Wang, C.C.; Wen, Z.; Zhu, Y.F.; Zhao, M.; Li, J.C.; Zheng, W.T.; Lian, J.S.; Jiang, Q. Design of hydrogen storage alloys/nanoporous metals hybrid electrodes for nickel-metal hydride batteries. Sci. Rep. 2016, 6, 27601.
- Giza, K.; Drulis, H. Effect of preparation method of metal hydride electrode on efficiency of hydrogen electrosorption process. Int. J. Mater. Res. 2016, 107, 103–108.
- Patelli, N.; Migliori, A.; Morandi, V.; Pasquini, L. Interfaces within biphasic nanoparticles give a boost to magnesium-based hydrogen storage. Nano Energy 2020, 72, 104654.
- Wang, Y.; Wang, Y. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. Mater. Int. 2017, 27, 41–49.
- Liu, G.; Wang, Y.; Qiu, F.; Li, L.; Jiao, L.; Yuan, H. Synthesis of porous nanocomposite and its synergetic effect on hydrogen sorption properties of MgH2. J. Mater. Chem. 2012, 22, 22542.
- Liu, M.; Xiao, X.; Zhao, S.; Saremi-Yarahmadi, S.; Chen, M.; Zheng, J.; Li, S.; Chen, L. ZIF-67 derived nanoparticles: Remarkably improved hydrogen storage properties of MgH2 and synergetic catalysis mechanism. Int. J. Hydrogen Energy 2019, 44, 1059–1069.
- Bhatnagar, A.; Pandey, S.K.; Vishwakarma, A.K.; Singh, S.; Shukla, V.; Soni, P.K.; Shaz, M.A.; Srivastava, O.N. Fe3O4@graphene as a superior catalyst for hydrogen de/absorption from/in MgH2/Mg. J. Mater. Chem. A 2016, 4, 14761–14772.
- Zhang, Y.; Wu, F.; Guemou, S.; Yu, H.; Zhang, L.; Wang, Y. Constructing Mg2Co-Mg2CoH5 nano hydrogen pumps from LiCoO2 nanosheets for boosting the hydrogen storage property of MgH2. Dalton Trans. 2022, 51, 16195–16205.
- Herrich, M.; Ismail, N.; Lyubina, J.; Handstein, A.; Pratt, A.; Gutfleisch, O. Synthesis and decomposition of Mg2FeH6 prepared by reactive milling. Mater. Sci. Eng. B 2004, 108, 28–32.
- Zhang, Y.; Wei, X.; Zhang, W.; Yuan, Z.; Gao, J.; Qi, Y.; Ren, H. Effect of milling duration on hydrogen storage thermodynamics and kinetics of Mg-based alloy. Int. J. Hydrogen Energy 2020, 45, 33832–33845.
- Choi, Y.J.; Choi, J.W.; Sohn, H.Y.; Ryu, T.; Hwang, K.S.; Fang, Z.Z. Chemical vapor synthesis of Mg–Ti nanopowder mixture as a hydrogen storage material. Int. J. Hydrogen Energy 2009, 34, 7700–7706.
- Cui, J.; Wang, H.; Liu, J.; Ouyang, L.; Zhang, Q.; Sun, D.; Yao, X.; Zhu, M. Remarkable enhancement in dehydrogenation of MgH2 by a nano-coating of multi-valence Ti-based catalysts. J. Mater. Chem. A 2013, 1, 5603–5611.
- Zou, J.; Zeng, X.; Ying, Y.; Chen, X.; Guo, H.; Zhou, S.; Ding, W. Study on the hydrogen storage properties of core-shell structured Mg–RE (RE = Nd, Gd, Er) nano-composites synthesized through arc plasma method. Int. J. Hydrogen Energy 2013, 38, 2337–2346.
- Jeon, K.J.; Moon, H.R.; Ruminski, A.M.; Jiang, B.; Kisielowski, C.; Bardhan, R.; Urban, J.J. Air-stable magnesium nanocomposites provide rapid and high-capacity hydrogen storage without using heavy-metal catalysts. Nat. Mater. 2011, 10, 286–290.
- Mao, J.; Zou, J.; Lu, C.; Zeng, X.; Ding, W. Hydrogen storage and hydrolysis properties of core-shell structured Mg-MFx (M=V, Ni, La and Ce) nano-composites prepared by arc plasma method. J. Power Sources 2017, 366, 131–142.
- Lu, C.; Zou, J.; Zeng, X.; Ding, W. Hydrogen storage properties of core-shell structured (TM=Co, V) composites. Int. J. Hydrogen Energy 2017, 42, 15246–15255.
