Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Wendy Huang and Version 1 by Rashed Abualia.
As a major component of vital macromolecules such as nucleic acids, amino acids, and chlorophyll, nitrogen is an essential macronutrient for plants. Although nitrogen is one of the most abundant elements in nature, accounting for about 70% of atmospheric gasses, its availability for plant uptake in the soil varies temporally and spatially. Therefore, modern agriculture relies heavily on nitrogen fertilization to maximize crop quality and yield. Numerous studies demonstrated that adaptation responses driven by nitrate/nitrogen are fine-tuned in concert with phytohormones, the endogenous signaling molecules that coordinate nearly every aspect of plant growth and development.
- nitrate
- auxin
- nitrogen
- plant growth and development
1. Introduction
Plants take up nitrogen from the soil in inorganic forms, such as nitrate and ammonium, or in organic forms, such as amino acids and peptides. Nitrate is the predominant form of nitrogen in aerobic soils [5][1] and the preferred nitrogen source for most higher plants, including Arabidopsis thaliana [6,7][2][3]. The acquisition of scarce nutrients such as nitrogen from the soil is one of the most challenging aspects of plant adaptation to a sessile lifestyle. Plants must cope with the varying availability and source compounds of this element and ensure its optimal uptake into the plant body. This is achieved by adjusting mechanisms and pathways that mediate soil exploration, nitrogen uptake, and distribution within the plant body [8][4]. The vital function of effective soil utilization and balanced uptake of the nitrogen-containing compounds is executed by the root organ. In the soil, the root system perceives and integrates local and systemic signals about the nitrogen status of the plant to regulate the uptake and distribution of nitrogen. An important component of this nutrient management strategy is flexible modulation of the root system architecture.
For example, after a period of deficiency, nitrate supply stimulates primary and lateral root growth and expansion [9[5][6][7][8],10,11,12], while supra-optimal nitrate levels have a negative effect on primary and lateral root growth [13,14,15][9][10][11]. The lateral roots of plants growing under heterogeneous nitrate conditions preferentially expand and colonize nitrate-rich zones [13][9]. Ammonium as the sole source of nitrogen suppresses the growth of primary and lateral roots [15][11] whereas L-glutamate as an organic nitrogen source inhibits the growth of primary roots but stimulates the growth of lateral roots [16][12]. This exceptional plasticity of the root system is at the core of nitrogen foraging, the ability of the root to adjust its growth and development to maximize nitrogen uptake under low and fluctuating nitrogen conditions.
Numerous recent studies demonstrated that adaptation responses driven by nitrate/nitrogen are fine-tuned in concert with phytohormones, the endogenous signaling molecules that coordinate nearly every aspect of plant growth and development. Hormone metabolite profiling [14,17,18[10][13][14][15][16],19,20], as well as a spectrum of omics approaches [21,22,23,24,25][17][18][19][20][21] clearly indicated close interactions between hormonal regulatory networks and pathways controlling nitrogen status. The expression of genes involved in biosynthesis, metabolism, transport, or signal transduction of plant hormones such as auxin, cytokinin, ethylene, abscisic acid, and gibberellins are rapidly modulated in plants exposed to fluctuating nitrogen conditions. NIN-like protein 7 (NLP7), the nitrate master regulator [22,24,26,27][18][20][22][23] and a recently reported intracellular nitrate sensor [28][24], was found to regulate components of hormonal regulatory networks [22,24,27,29][18][20][23][25]. NRT1.1, a well-established nitrate transceptor, was shown to adjust levels and distribution of auxin to low nitrate levels by regulating its biosynthesis and transport [30,31,32][26][27][28]. The inhibitory effects of excessive nitrate supply on root growth and branching were associated with an increase in abscisic acid and ethylene biosynthesis via increased expression of the corresponding biosynthetic genes such as ABA1, ABA2, and ABA3 [33][29] and ACS [14][10]. These examples demonstrate that plant hormones are important endogenous integrators and translators of nitrogen status to plant adaptive responses.
2. The Role of Auxin in Nitrate-Regulated Plant Growth and Development
Auxins are a group of naturally occurring molecules derived from tryptophan, with indole-3-acetic acid (IAA) being the major form of auxin. The biosynthesis of IAA is defined by a two-step metabolic pathway, in which the TAA family of aminotransferases converts tryptophan (Trp) to indole-3-pyruvate (IPA), followed by a YUC flavin monooxygenases-mediated conversion of IPA to IAA [58][30]. Auxin has extensive regulatory functions in plant development, including tropic responses, embryogenesis, and postembryonic initiation and formation of organs [59,60,61][31][32][33]. The auxin signal transduction cascade is activated by the hormone-triggered interaction of the auxin receptor SCFTIR1/AFB E3 ubiquitin ligase with Aux/IAA signaling repressors, which leads to the latter’s polyubiquitination and degradation by the proteasome. Consequently, transcription factors of the Auxin Response Factor (ARF) family are relieved from inhibition by Aux/IAAs and transcription of auxin-responsive genes is promoted [62][34]. In Arabidopsis, there are 23 ARFs displaying differential affinities to members of the Aux/IAA repressor family, which encompasses 29 homologues [62,63][34][35]. Variable homo- and hetero-oligomerizations of Aux/IAAs may provide an additional mechanism for the diversity of the auxin response [63,64][35][36]. Beyond the canonical auxin signal transduction cascade, revolving around TIR1/AFB-Aux/IAA-ARF, observations of auxin-triggered rapid non-transcriptional growth responses suggest another auxin receptor/sensor might operate in planta [65,66][37][38]. Recently, ABP1 and the auxin signaling proteins of the transmembrane kinase (TMK) family were shown to interact with plasma membrane H+-ATPases, inducing their phosphorylation and thereby promoting cell wall acidification and rapid elongation of hypocotyl cells in Arabidopsis [67,68,69][39][40][41]. Besides auxin metabolism, perception and signal transduction, the tightly controlled transport machinery is another key component of the regulatory system determining the biological activity of auxin. In higher plants, auxin is transported from young leaves to roots via the phloem vasculature [70][42]. This long-distance auxin transport is complemented by polar auxin transport (PAT), mediating cell-to-cell transport of the hormone [71,72][43][44]. This slower mode of auxin transport depends on active auxin influx and efflux between cells and is of great biological importance as it enables the directional movement of auxin as well as distribution gradients across tissues and organs. PAT is mediated by several families of membrane transporters including AUX1/LIKE AUX (AUX/LAX), PIN-FORMED(PIN), PIN-LIKES (PILS), and ATB Binding Cassette B (ABCB) [30,73,74,75,76][26][45][46][47][48]. Considering the importance of auxin in plant growth, developmental and physiological processes, it is not surprising that the investigation of its role in adaptation to nitrogen sources and in particular to nitrate availability has become one of the major research foci over the last decades. Early experiments conducted in the 1930s and 1940s showed that the auxin content in shoots of Brassica caulorapa and other species is dependent on the amount of supplied nitrate [77][49]. Since then, numerous works have pointed out that auxin biosynthesis, transport and signaling pathways are important mechanisms underlying plant growth and developmental adaptation to varying levels and sources of nitrogen [9,19,30,31,32,78,79,80][5][15][26][27][28][50][51][52]. A study by Ma et al. [81][53] showed that expression of key components of auxin biosynthesis including tryptophan aminotransferase 1 (TAR1), TAR2, and their close homologs TAA1 is regulated by nitrogen availability. Among them, TAR2 was found to play a critical function in maintaining auxin levels and fine-tuning lateral root outgrowth under mild nitrogen-limiting conditions [81][53]. TAR2 expression is controlled by NRT1.1, which acts as a negative regulator under nitrate depletion conditions. The suppression of TAR2 transcription is abolished either by the provision of nitrate or in nrt1.1 mutant [31][27]. Collectively, these studies demonstrate how nitrate contributes to fine-tuning lateral root outgrowth and adjusting it to fluctuating nitrate availability via TAR2-mediated biosynthesis of auxin in the root stele. Identification of several components of the PAT machinery including PIN1, PIN2, PIN4 and PIN7 in the nitrate-responsive transcriptome suggested that the distribution of auxin in the plant body is controlled by nitrate [25][21]. This conclusion has been confirmed by Maghiaoui et al. [31][27], who demonstrated that mRNA levels of PIN1, PIN4, PIN7, but also ABCB4, ABCB19 auxin transporters are modulated by nitrate—independently of NRT1.1 perception however, thus raising a question about the molecular bases of this regulatory network. Nitrate-regulated transcription of auxin influx carriers such as AUX1 and LAX3 on the other hand, is dependent on NRT1.1 [31][27] and plays an important role in adjusting lateral root outgrowth to nitrate availability. Intriguingly, in addition to the well-established components of PAT such as PINs, AUX/LAX and ABCB transporters also NRT1.1, initially identified as a dual nitrate transporter, was found to transport auxin [30,82][26][54]. The auxin transport activity of NRT1.1 turned out to be particularly important for adjusting root branching to nitrate availability. Under low nitrate conditions, NRT1.1 transports auxin away from the tip of the lateral root primordium (LRP), which ultimately results in its developmental arrest [30][26]. Taken together, the NRT1.1 transceptor coordinates auxin-dependent development of LRPs via local control of auxin synthesis, redistribution of auxin in the primordium, and fine-tuning expression of LAX3 in the tissue overlying the LRP. There, the LAX3 influx driven accumulation of auxin controls cell wall loosening which allows the LRP to emerge through adjacent tissues (Figure 1A) [30,31,83,84][26][27][55][56].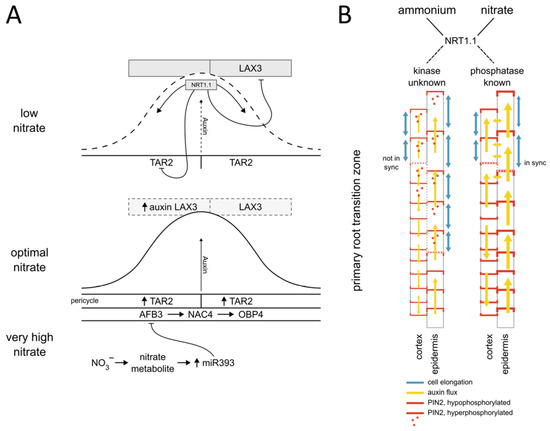
Figure 1. Nitrate modulated auxin synthesis, transport, and signaling converge at regulation of the lateral root primordia (LRP) development and Primary root growth. (A) At low nitrate levels NRT1.1 controls the LRP development through transporting auxin and by suppressing TAR2-mediated auxin biosynthesis and LAX3-dependent influx of auxin to cells adjacent to the LRP. The nitrate-dependent expression of the auxin receptor AFB3 is part of the regulatory module controlling LRP development. Under optimal nitrate conditions AFB3 mediates the expression of NAC4 and OBP4 transcription factors, while nitrate metabolite-induced expression of miR393 suppresses AFB3, thus providing a negative feedback loop. (B) Nitrogen source-dependent phosphorylation of PIN2 determines the membrane localization of this auxin transporter in epidermal and cortex cells at the primary root (transition zone depicted). The fine-tuning of PIN2 levels at the plasma membrane regulates the auxin flux between two adjacent tissue layers (yellow arrows), thereby coordinating cell elongation patterns (Modified from [9][5]).
References
- von Wirén, N.; Gazzarrini, S.; Gojon, A.; Frommer, W.B. The molecular physiology of ammonium uptake and retrieval. Curr. Opin. Plant Biol. 2000, 3, 254–261.
- Crawford, N.M.; Glass, A.D.M. Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 1998, 3, 389–395.
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48.
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266.
- Ötvös, K.; Marconi, M.; Vega, A.; O’Brien, J.; Johnson, A.; Abualia, R.; Antonielli, L.; Montesinos, J.C.; Zhang, Y.; Tan, S.; et al. Modulation of plant root growth by nitrogen source-defined regulation of polar auxin transport. EMBO J. 2021, 40, e106862.
- Alvarez, J.M.; Riveras, E.; Vidal, E.A.; Gras, D.E.; Contreras-López, O.; Tamayo, K.P.; Aceituno, F.; Gómez, I.; Ruffel, S.; Lejay, L.; et al. Systems approach identifies TGA1 and TGA4 transcription factors as important regulatory components of the nitrate response of Arabidopsis thaliana roots. Plant J. Cell Mol. Biol. 2014, 80, 1–13.
- Vidal, E.A.; Araus, V.; Lu, C.; Parry, G.; Green, P.J.; Coruzzi, G.M.; Gutiérrez, R.A. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 4477–4482.
- Vidal, E.A.; Álvarez, J.M.; Gutiérrez, R.A. Nitrate regulation of AFB3 and NAC4 gene expression in Arabidopsis roots depends on NRT1.1 nitrate transport function. Plant Signal. Behav. 2014, 9, e28501.
- Zhang, H.; Forde, B.G. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 1998, 279, 407–409.
- Tian, Q.-Y.; Sun, P.; Zhang, W.-H. Ethylene is involved in nitrate-dependent root growth and branching in Arabidopsis thaliana. New Phytol. 2009, 184, 918–931.
- Li, Q.; Li, B.-H.; Kronzucker, H.J.; Shi, W.-M. Root growth inhibition by NH(4)(+) in Arabidopsis is mediated by the root tip and is linked to NH(4)(+) efflux and GMPase activity. Plant Cell Environ. 2010, 33, 1529–1542.
- Walch-Liu, P.; Ivanov, I.I.; Filleur, S.; Gan, Y.; Remans, T.; Forde, B.G. Nitrogen Regulation of Root Branching. Ann. Bot. 2006, 97, 875–881.
- Takei, K.; Ueda, N.; Aoki, K.; Kuromori, T.; Hirayama, T.; Shinozaki, K.; Yamaya, T.; Sakakibara, H. AtIPT3 is a Key Determinant of Nitrate-Dependent Cytokinin Biosynthesis in Arabidopsis. Plant Cell Physiol. 2004, 45, 1053–1062.
- Poitout, A.; Crabos, A.; Petřík, I.; Novák, O.; Krouk, G.; Lacombe, B.; Ruffel, S. Responses to Systemic Nitrogen Signaling in Arabidopsis Roots Involve trans-Zeatin in Shoots. Plant Cell 2018, 30, 1243–1257.
- Abualia, R.; Ötvös, K.; Novák, O.; Bouguyon, E.; Domanegg, K.; Krapp, A.; Nacry, P.; Gojon, A.; Lacombe, B.; Benková, E. Molecular framework integrating nitrate sensing in root and auxin-guided shoot adaptive responses. Proc. Natl. Acad. Sci. USA 2022, 119, e2122460119.
- Maeda, Y.; Konishi, M.; Kiba, T.; Sakuraba, Y.; Sawaki, N.; Kurai, T.; Ueda, Y.; Sakakibara, H.; Yanagisawa, S. A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat. Commun. 2018, 9, 1376.
- Varala, K.; Marshall-Colón, A.; Cirrone, J.; Brooks, M.D.; Pasquino, A.V.; Léran, S.; Mittal, S.; Rock, T.M.; Edwards, M.B.; Kim, G.J.; et al. Temporal transcriptional logic of dynamic regulatory networks underlying nitrogen signaling and use in plants. Proc. Natl. Acad. Sci. USA 2018, 115, 6494–6499.
- Alvarez, J.M.; Schinke, A.-L.; Brooks, M.D.; Pasquino, A.; Leonelli, L.; Varala, K.; Safi, A.; Krouk, G.; Krapp, A.; Coruzzi, G.M. Transient genome-wide interactions of the master transcription factor NLP7 initiate a rapid nitrogen-response cascade. Nat. Commun. 2020, 11, 1157.
- Vega, A.; Fredes, I.; O’Brien, J.; Shen, Z.; Ötvös, K.; Abualia, R.; Benkova, E.; Briggs, S.P.; Gutiérrez, R.A. Nitrate triggered phosphoproteome changes and a PIN2 phosphosite modulating root system architecture. EMBO Rep. 2021, 22, e51813.
- Liu, K.; Niu, Y.; Konishi, M.; Wu, Y.; Du, H.; Sun Chung, H.; Li, L.; Boudsocq, M.; McCormack, M.; Maekawa, S.; et al. Discovery of nitrate–CPK–NLP signalling in central nutrient–growth networks. Nature 2017, 545, 311–316.
- Gutiérrez, R.A.; Lejay, L.V.; Dean, A.; Chiaromonte, F.; Shasha, D.E.; Coruzzi, G.M. Qualitative network models and genome-wide expression data define carbon/nitrogen-responsive molecular machines in Arabidopsis. Genome Biol. 2007, 8, R7.
- Castaings, L.; Camargo, A.; Pocholle, D.; Gaudon, V.; Texier, Y.; Boutet-Mercey, S.; Taconnat, L.; Renou, J.-P.; Daniel-Vedele, F.; Fernandez, E.; et al. The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. Plant J. 2009, 57, 426–435.
- Marchive, C.; Roudier, F.; Castaings, L.; Bréhaut, V.; Blondet, E.; Colot, V.; Meyer, C.; Krapp, A. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat. Commun. 2013, 4, 1713.
- Liu, K.-H.; Liu, M.; Lin, Z.; Wang, Z.-F.; Chen, B.; Liu, C.; Guo, A.; Konishi, M.; Yanagisawa, S.; Wagner, G.; et al. NIN-like protein 7 transcription factor is a plant nitrate sensor. Science 2022, 377, 1419–1425.
- Liu, K.-H.; Diener, A.; Lin, Z.; Liu, C.; Sheen, J. Primary nitrate responses mediated by calcium signalling and diverse protein phosphorylation. J. Exp. Bot. 2020, 71, 4428–4441.
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937.
- Maghiaoui, A.; Bouguyon, E.; Cuesta, C.; Perrine-Walker, F.; Alcon, C.; Krouk, G.; Benková, E.; Nacry, P.; Gojon, A.; Bach, L. The Arabidopsis NRT1.1 transceptor coordinately controls auxin biosynthesis and transport to regulate root branching in response to nitrate. J. Exp. Bot. 2020, 71, 4480–4494.
- Mounier, E.; Pervent, M.; Ljung, K.; Gojon, A.; Nacry, P. Auxin-mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant Cell Environ. 2014, 37, 162–174.
- Signora, L.; De Smet, I.; Foyer, C.H.; Zhang, H. ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. Plant J. Cell Mol. Biol. 2001, 28, 655–662.
- Zhao, Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol. Plant 2012, 5, 334–338.
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell 2003, 115, 591–602.
- Friml, J.; Vieten, A.; Sauer, M.; Weijers, D.; Schwarz, H.; Hamann, T.; Offringa, R.; Jürgens, G. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature 2003, 426, 147–153.
- Reinhardt, D.; Pesce, E.-R.; Stieger, P.; Mandel, T.; Baltensperger, K.; Bennett, M.; Traas, J.; Friml, J.; Kuhlemeier, C. Regulation of phyllotaxis by polar auxin transport. Nature 2003, 426, 255–260.
- Chapman, E.J.; Estelle, M. Mechanism of auxin-regulated gene expression in plants. Annu. Rev. Genet. 2009, 43, 265–285.
- Rademacher, E.H.; Lokerse, A.S.; Schlereth, A.; Llavata-Peris, C.I.; Bayer, M.; Kientz, M.; Freire Rios, A.; Borst, J.W.; Lukowitz, W.; Jürgens, G.; et al. Different Auxin Response Machineries Control Distinct Cell Fates in the Early Plant Embryo. Dev. Cell 2012, 22, 211–222.
- Bargmann, B.O.R.; Vanneste, S.; Krouk, G.; Nawy, T.; Efroni, I.; Shani, E.; Choe, G.; Friml, J.; Bergmann, D.C.; Estelle, M.; et al. A map of cell type-specific auxin responses. Mol. Syst. Biol. 2013, 9, 688.
- Fendrych, M.; Akhmanova, M.; Merrin, J.; Glanc, M.; Hagihara, S.; Takahashi, K.; Uchida, N.; Torii, K.U.; Friml, J. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nat. Plants 2018, 4, 453–459.
- Kubeš, M.; Napier, R. Non-canonical auxin signalling: Fast and curious. J. Exp. Bot. 2019, 70, 2609–2614.
- Lin, W.; Zhou, X.; Tang, W.; Takahashi, K.; Pan, X.; Dai, J.; Ren, H.; Zhu, X.; Pan, S.; Zheng, H.; et al. TMK-based cell-surface auxin signalling activates cell-wall acidification. Nature 2021, 599, 278–282.
- Li, L.; Verstraeten, I.; Roosjen, M.; Takahashi, K.; Rodriguez, L.; Merrin, J.; Chen, J.; Shabala, L.; Smet, W.; Ren, H.; et al. Antagonistic cell surface and intracellular auxin signalling regulate plasma membrane H+-fluxes for root growth. Nature 2021, 599, 273.
- Friml, J.; Gallei, M.; Gelová, Z.; Johnson, A.; Mazur, E.; Monzer, A.; Rodriguez, L.; Roosjen, M.; Verstraeten, I.; Živanović, B.D.; et al. ABP1–TMK auxin perception for global phosphorylation and auxin canalization. Nature 2022, 609, 575–581.
- Cambridge, A.P.; Morris, D.A. Transfer of exogenous auxin from the phloem to the polar auxin transport pathway in pea (Pisum sativum L.). Planta 1996, 199, 583–588.
- Adamowski, M.; Friml, J. PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 2015, 27, 20–32.
- Abualia, R.; Benkova, E.; Lacombe, B. Transporters and Mechanisms of Hormone Transport in Arabidopsis. Adv. Bot. Res. 2018, 87, 342.
- Barbez, E.; Kubeš, M.; Rolčík, J.; Béziat, C.; Pěnčík, A.; Wang, B.; Rosquete, M.R.; Zhu, J.; Dobrev, P.I.; Lee, Y.; et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 2012, 485, 119–122.
- Okada, K.; Ueda, J.; Komaki, M.K.; Bell, C.J.; Shimura, Y. Requirement of the Auxin Polar Transport System in Early Stages of Arabidopsis Floral Bud Formation. Plant Cell 1991, 3, 677–684.
- Bennett, M.J.; Marchant, A.; Green, H.G.; May, S.T.; Ward, S.P.; Millner, P.A.; Walker, A.R.; Schulz, B.; Feldmann, K.A. Arabidopsis AUX1 Gene: A Permease-Like Regulator of Root Gravitropism. Science 1996, 273, 948–950.
- Ye, L.; Liu, L.; Xing, A.; Kang, D. Characterization of a dwarf mutant allele of Arabidopsis MDR-like ABC transporter AtPGP1 gene. Biochem. Biophys. Res. Commun. 2013, 441, 782–786.
- Avery, G.S.; Pottorf, L. Auxin and Nitrogen Relationships in Green Plants. Am. J. Bot. 1945, 32, 666–669.
- Caba, J.M.; Centeno, M.L.; Fernández, B.; Gresshoff, P.M.; Ligero, F. Inoculation and nitrate alter phytohormone levels in soybean roots: Differences between a supernodulating mutant and the wild type. Planta 2000, 211, 98–104.
- Tian, Q.; Chen, F.; Liu, J.; Zhang, F.; Mi, G. Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J. Plant Physiol. 2008, 165, 942–951.
- Meier, M.; Liu, Y.; Lay-Pruitt, K.S.; Takahashi, H.; von Wirén, N. Auxin-mediated root branching is determined by the form of available nitrogen. Nat. Plants 2020, 6, 1136–1145.
- Ma, W.; Li, J.; Qu, B.; He, X.; Zhao, X.; Li, B.; Fu, X.; Tong, Y. Auxin biosynthetic gene TAR2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. Plant J. Cell Mol. Biol. 2014, 78, 70–79.
- Bouguyon, E.; Brun, F.; Meynard, D.; Kubeš, M.; Pervent, M.; Leran, S.; Lacombe, B.; Krouk, G.; Guiderdoni, E.; Zažímalová, E.; et al. Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1.1. Nat. Plants 2015, 1, 15015.
- Porco, S.; Larrieu, A.; Du, Y.; Gaudinier, A.; Goh, T.; Swarup, K.; Swarup, R.; Kuempers, B.; Bishopp, A.; Lavenus, J.; et al. Lateral root emergence in Arabidopsis is dependent on transcription factor LBD29 regulation of auxin influx carrier LAX3. Development 2016, 143, 3340–3349.
- Lay-Pruitt, K.S.; Takahashi, H. Integrating N signals and root growth: The role of nitrate transceptor NRT1.1 in auxin-mediated lateral root development. J. Exp. Bot. 2020, 71, 4365–4368.
- Lee, H.; Ganguly, A.; Baik, S.; Cho, H.-T. Calcium-dependent protein kinase 29 modulates PIN-FORMED polarity and Arabidopsis development via its own phosphorylation code. Plant Cell 2021, 33, 3513–3531.
More
