Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Abdur Rashid and Version 2 by Conner Chen.
Heavy metals and metalloids (HMs) are environmental pollutants, most notably cadmium, lead, arsenic, mercury, and chromium. When HMs accumulate to toxic levels in agricultural soils, these non-biodegradable elements adversely affect crop health and productivity. The toxicity of HMs on crops depends upon factors including crop type, growth condition, and developmental stage; nature of toxicity of the specific elements involved; soil physical and chemical properties; occurrence and bioavailability of HM ions in the soil solution; and soil rhizosphere chemistry. HMs can disrupt the normal structure and function of cellular components and impede various metabolic and developmental processes.
- heavy metals
- arable lands
- agricultural practices
1. Introduction
Metals, including potentially toxic elements, are inorganic elements containing atomic densities (g·cm−3) several times higher than H2O (1 g·cm−3) and broadly classified into heavy and light metals, and semi-metals (Figure 1). Based on physical, physiological, and chemical properties, metals have been classified under several sub-groups, namely: transition metals: e.g., chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and molybdenum (Mo); post-transition metals: e.g., aluminum (Al), zinc (Zn), cadmium (Cd), mercury (Hg), and lead (Pb); alkaline earth metals: e.g., calcium (Ca), magnesium (Mg), beryllium (Be), and barium (Ba); alkali metals: e.g., lithium (Li), sodium (Na), potassium (K), and cesium (Cs); and metalloids, which are also referred to as semi-metals because of their metallic and non-metallic properties: e.g., boron (B), silicon (Si), arsenic (As), and antimony (Sb) [1].
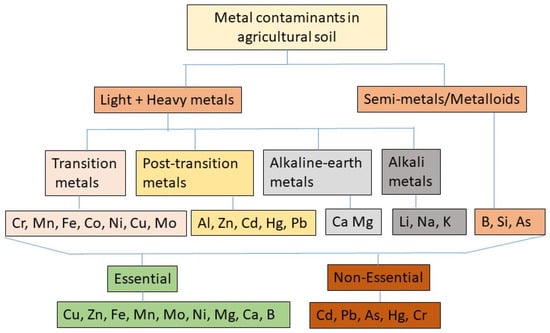
Figure 1. Classification of metallic and non-metallic elements frequently found in agricultural soils. Mg (magnesium), Ca (calcium), Fe (iron), B (boron), Mn (manganese), Zn (zinc), Mo (molybdenum), Cu (copper), Pb (lead), Ni (nickel), Cr (chromium), As (arsenic), Hg (mercury), Cd (cadmium), Al (aluminum), Li (lithium), K (potassium), Na (sodium), Si (silicon).
Heavy metals and metalloids (HMs) are environmental pollutants. They are also agricultural soil contaminants, because if present at elevated levels in the soil, HMs can negatively impact crop health and productivity [2][3][2,3]. HMs are recalcitrant to degradation, and if not taken up by plants or removed by leaching, they can accumulate in the soil and persist for long periods [4][5][6][4,5,6]. The elements that are frequently found to contaminate agricultural soils and cause toxic effects at elevated levels on plants include Cd, Pb, Cr, As, Hg, Ni, Cu, and Zn [4][7][4,7]. Among them, Cd, Pb, As, Hg, and Cr are highly toxic and detrimental to plant health at almost all levels of contamination [8][9][10][8,9,10].
Several elements are classified as essential mineral nutrients for plant growth and productivity (Figure 1). Examples include Cu, Zn, Fe, Mn, Mo, Ni, Mg, Ca, and B. At relatively low concentrations, these elements can enhance specific cellular functions in plants including ion homeostasis, pigment biosynthesis, photosynthesis, respiration, enzyme activities, gene regulation, sugar metabolism, nitrogen fixation, etc. [3][8][11][3,8,11]. However, when accumulated at concentrations above optimum, these same essential elements can adversely affect plant growth, development, and reproduction [2][3][2,3]. Conversely, if the concentration falls below certain threshold levels, they also produce mineral deficiency symptoms in plants [11].
HM contamination in agricultural soil is a global issue. In addition to certain geogenic and climatic factors, specific circumstances such as rapid urbanization, and increased industrial, municipal, agricultural, domestic, medical, and technological applications appear to be the major causes of HM pollution in the environment at the present time. However, the problem is more prominent in many developing countries, partly because of the above reasons, and perhaps partly due to a lack of proper awareness about the toxic consequences of these elements not only to human health but also to crop health [12][13][14][15][16][12,13,14,15,16].
2. Sources of HM Contamination in Arable Lands
Agricultural soil is an important non-renewable natural resource. It can be contaminated with toxic HM elements such as Cd, Pb, Cr, As, Hg, Cu, Ni, Zn, Al, and several others due to natural causes as well as anthropogenic activities. Natural causes include, among many others, weathering of metal-bearing rocks by rainwater and atmospheric deposition. Anthropogenic activities include industrial activities (e.g., mining, leather tanning, textile, and petrol-chemical), disposal of metal-containing wastes, vehicle exhausts, and agricultural practices [4][15][17][18][19][20][4,15,17,18,19,20]. However, irrespective of the source of contamination, continued addition of HMs to arable lands can result in soils that can be too toxic to support plant growth and productivity.2.1. Application of Chemical Fertilizers
Chemical fertilizers, particularly inorganic fertilizers, are a crucial input for crop production. Consequently, large quantities of inorganic fertilizers, including nitrogen (N), phosphorus (P), potassium (K), and compound/mixed fertilizers are routinely added to agricultural lands to supply adequate quantities of these macronutrients. For instance, it was estimated that in 2019, more than 220 million tons of commercial fertilizers and liming materials were applied worldwide, mostly to agricultural fields [21]. Among these fertilizers, P fertilizers contain the highest level of HM contaminants [4][22][23][24][4,22,23,24]. For example, superphosphate fertilizers can contain Cd, Co, Cu, Pb, Zn, Cr, and Ni as contaminants. In a study that assessed soil with and without P fertilizer amendments, the concentration of Zn was higher not only in the amended soil, but also in the plants grown in that soil [25]. Cadmium content in the soil has been shown to increase persistently due to the application of P fertilizers [12][23][26][12,23,26]. Cadmium is extremely toxic to plants because of its high solubility and mobility in soil solution. The concentration of Cd present as an impurity in several P fertilizers evaluated in a study is shown in Table 1.Table 1.
| Fertilizers | Cadmium Content (mg Kg−1) | |
|---|---|---|
| Based on Product | Based on P Content | |
| Complete fertilizer | 23–29 | 418–527 |
| Single superphosphate | 16–26 | 186–302 |
| Superphosphate | 13–34 | 151–395 |
| Rock phosphate | ||
| Insecticides | ||
| Alnuwaiser | ||
| [ | ||
| 39 | ||
| ] | ||
| Sniper | ||
| ® | ||
| Fipronil | ||
| Zn (506), Cu (423), Cr (746), Co (275), Pb (88) | ||
| CyperCel | ||
| ® | ||
| Cypermethrin | ||
| Zn (2389), Cu (669), Cr (373), Co (18), Pb (807) | ||
| CyperSafe® | Cypermethrin | Zn (968), Cu (464), Cr (10), Co (6), Pb (119) |
| Scope 60® | Asaybrmthrin | Zn (527), Cu (539), Cr (437), Co (23), Pb (39) |
| Brodor® | Permethrin | Zn (10), Cr (16), Pb (186) |
| Clash® | Acephate + Buprofezin | Zn (1078), Cr (73), Co (39), Pb (1316) |
| Acefed® | Mithomail | Cu (19), Cr (48), Co (4), Pb (121) |
| Lanid® | - | Cu (128), Cr (60), Pb (98) |
| Probalt® | - | Cu (179), Cr (85), Co (25), Pb (46) |
| Nourcam® | - | - |
| Madar® | - | Zn (10), Cu (66), Cr (16), Co (10), |
| PifPaf® | - | Cu (110), Co (5), Thallium, Tl (19), Pb (12) |
| Paygon® | - | Zn (52), Tl (15), Pb (19) |
* European Union (EU)/World Health Organization (WHO) prescribed permissible levels (ppb) in water: As (10), Cr (50), Ni (20/70), Pb (10), Co (NA).
Several fungicides and insecticides extensively used in the past in agricultural lands were shown to contain significant concentrations of HM elements in their active ingredients. Examples include Cu-containing fungicides such as copper sulphate (Bordeaux mixture, also referred to as Bordo® mix) and copper oxychloride; Pb-containing insecticide such as lead arsenate; and Cu-containing insecticide such as copper acetoarsenite. The commonly found HM elements in the active ingredients of pesticide products include Cu, As, Pb, Hg, Cr, Zn, Al, Li, Ba, B, and Ti (titanium) [36][37][36,37].
On the other hand, HM elements can also be present in pesticide products as impurities. For example, certain pesticide products used for pest control in Japan contained Cd, Hg, As, Cu, Zn, and Pb as contaminants [17]. A chemical analysis of several pesticides, including 11 glyphosate-based herbicide formulations, by utilizing inductively coupled plasma/optical emission spectrometry (ICP-OES) detected As, Cr, Co, Pb, and Ni as contaminants [38]. A similar analysis of several insecticides using ICP mass spectrometry detected Zn, Cu, Cr, Co, Pb, and Tl (thallium) as contaminants [39]. It has been suggested that the HM elements contaminate pesticide products during the manufacturing process, while some of them are intentionally added as nano pesticides for increased efficacy [38][40][38,40].
In addition to P fertilizers, copper sulphate, iron sulphate, and zinc sulphate fertilizers can also contain HM contaminants, including Pb [22][27][28][22,27,28]. A study reported from greenhouse experiments that repeated application of chemical fertilizers significantly increased the accumulation of several HM elements in the soil (Table 2). Experiments carried out with soil samples collected from multiple locations in agricultural lands of peninsular Malaysia and Guangdong Province of China show that the concentrations of different HM elements (As, Cd, Co, Cr, Cu, Hg, Ni, Pb, Zn) were severalfold higher as compared to control soil samples (refer to Table S2). These HM elements have originated from suspected agricultural practices, including fertilizer applications. Sources of HM contamination in fertilizers include the raw materials used in the manufacture of inorganic fertilizers. For instance, phosphate rock, also known as phosphorite, is utilized in the production of P fertilizers [29][30][29,30]. Over 90% of potash extracted from mines is used in the manufacture of K fertilizer [31]. Based on the level of HM impurities, chemical fertilizers can be ranked as follows: P fertilizers ≥ compound fertilizers> K fertilizers> N fertilizer [32][33][32,33].
56][57][9,15,16,56,57]. A review of many related articles published in a span of over two decades (1994 to 2019) that determined the HM contamination in surface water bodies throughout the world showed that the average content of Cr, Mn, Co, Ni, As, and Cd exceeded the permissible limits as prescribed by the WHO and the United States EPA [9][20][9,20]. Studies conducted to determine HM concentrations in irrigation water in several locations of the Gazipur district of Bangladesh and the Gondar city of Ethiopia showed that in almost all tests, the concentration of HMs exceeded the FAO (Food and Agriculture Organization) prescribed admissible levels (Table 7).
2.3. Application of Livestock Manures and Compost
Livestock manures are organic fertilizers composed predominantly of poultry, cattle, and pig manures. Application of these manures and the compost made from them to farmlands is a common practice in agricultural crop production. However, these manures and compost contain high concentrations of HM elements such as Cu, Zn, Cd, Ni, Cr, As, Pb, and Hg as contaminants [20][26][41][42][20,26,41,42]. A study conducted to determine the concentrations of HM elements in different livestock and poultry manures is presented in Table 5. The major sources of contamination of HM elements in the manures include the minerals supplied with the commercial feeds [41][42][41,42]. For example, supplementation of animal feeds with growth-promoting organic arsenical products was practiced for many years [43]. Some studies confirmed that Zn, Cu, As, and Cd were artificially added to commercial feeds to promote animal growth and improve disease resistance [44][45][44,45]. However, animals cannot digest these HM elements, and discharge them through manure [46]. Because HMs are non-degradable elements, they are also not broken down during the composting process [47]. Thus, long-term repeated applications of manures and compost can result in the buildup of HM elements to toxic levels in agricultural soil [48][49][48,49] and can affect crop health and productivity.Table 5. A case study showing maximum (MAX) vs. minimum (MIN) concentration (mg Kg−1 dry weight) of HM elements in different livestock and poultry manures, and their fold differences (FD). (Adapted from [42]).
| Source | Level | Zn | Cu | Pb | Cd | Cr | Hg | As | Ni |
|---|---|---|---|---|---|---|---|---|---|
| 7.2–47 | |||||||||
| 54–303 | |||||||||
| High analysis fertilizer | <0.6–5.6 | 15–118 | |||||||
| Double superphosphate | <0.6–12 | <3.6–72 | |||||||
| Triple superphosphate | 0.8–7.0 | 24–35 | |||||||
| Mono-ammonium phosphate | 1.8–8.1 | 12–37 | |||||||
| Di-ammonium phosphate | 4.3–6.6 | 22–28 |
Table 2. Heavy metal concentrations in greenhouse soil because of repeated application of inorganic fertilizers (adapted from [24]).
| Elements | MAX | MIN | Fold Difference |
|---|---|---|---|
| mg Kg−1 Soil | |||
| Cd | 0.65 | 0.06 | 10.8 |
| HgCl | |||
| 2 | |||
| Hg | |||
Table 4.
Pesticide products containing HM elements as impurities.
| Trade Name | Technical Name | Metal Impurities (ppb) * | |
|---|---|---|---|
| Insecticides | Defarge et al. | ||
| Cu | 171.5 | 21.0 | 8.2 |
| Ni | 36.9 | 28.7 | 1.3 |
| Pb | 38.0 | 20.5 | 1.9 |
| Zn | 433 | 71.9 | 6 |
2.2. Pesticide Application
Pesticides play an important role in global agriculture. It has been estimated that without pesticides, the world’s food production could be reduced by close to ~40% [34]. Another study estimated a 78% loss of fruit production, 54% loss of vegetable production, and 32% loss of cereal production without pesticide use [35]. Pesticides such as insecticides, fungicides, rodenticides, nematicides, and herbicides are composed of either organic or inorganic compounds that are toxic to the targeted organisms. Analysis of these compounds shows that some of them contain HM elements either as active ingredients (Table 3) or as impurities in the formulations (Table 4).Table 3. Pesticides containing different HM elements in their active ingredients (adapted from [36][37]).
| Chemical Name | Formula | HM Elements | |||
|---|---|---|---|---|---|
| Insecticides | |||||
| [ | 38 | ] | Aluminum phosphide | AIP | Al |
| Pyrinex® | Chlorpyriphos | As (390), Cr (800), Ni (1200) | Aluminum silicate | Al2Si2O7 | Al |
| Folpan | |||||
| (CH | |||||
| 0.6 | |||||
| 1.2 | |||||
| FD | |||||
| 10 | |||||
| 26 | |||||
| 12 | |||||
| 4.7 | |||||
| 2.8 | |||||
| 12 | 4.3 | 10 |
2.4. Application of Sewage-Sludge-Based Biosolids
Sewage sludge originated from municipal and industrial wastes can be highly contaminated with HM elements such as As, Cd, Cr, Cu, Pb, Hg, Ni, Mo, Zn, and others. Long-term application of untreated sewage sludge in some developing countries has led to increased concentrations of HMs in the agricultural lands [14][20][50][14,20,50]. However, the biosolids generated from sewage sludge processing plants can be typically low in HM contamination and can contain organic materials rich in nutrients, and be used as fertilizers [51][52][51,52]. When applied to arable lands, processed sewage sludge can improve soil physical properties and crop productivity. Utilization of these byproducts for agricultural crop production is, therefore, a common practice in many countries. In the United States, about 3.0 million dry tons of biosolids are utilized annually for crop production [4]. The European community countries utilized >30% of processed sewage sludge as a fertilizer in agricultural lands [53]. Australia incorporates over 175K tons of dry biosolids into agricultural soil [54]. In the United States, federal regulations limit concentrations of major elements (e.g., As, Cd, Cu, Pb, Hg, Ni, Se, and Zn) commonly found in biosolids for land application (Table 6) [52]. Although biosolids produced from sewage sludge processing treatment are generally low in HM concentration, repeated applications of these products can result in the buildup of HM elements in agricultural soil and can negatively impact crop health and productivity.Table 6.
Regulatory limits for HM elements, commonly found in applied biosolids in the USA (adapted from [55]).
| Elements | Maximum Permissible Level (mg Kg−1) | Cumulative Loading Rate (Kg ha−1) | Monthly Average Concentrations (mg Kg−1) | Annual Loading Rate (Kg ha−1) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pig | MAX | 4639 | 1288 | 23 | 60 | 85 | 0.3 | 89 | 19 | |||||||||
| As | 75 | 41 | 41 | 2.0 | ||||||||||||||
| (23.1) | 15.20 | (7.6) | 0.52 (5.2) |
0.02 (2.0) |
1.15 (0.2) |
- | Ahmed et al. [16] | MIN | Cd100 | |||||||||
| Max level | 85 | 73 | (FD)0.3 | 390.04 | 3.5 | 0.9439 | 0.0 |
0.01 | 4.7 | |||||||||
| (9.4) | 0.61 | 1.9 | ||||||||||||||||
| (3.1) | 0.86 | (0.4) |
- | 0.04 (4.0) |
0.19 (0.04) |
0.12 (0.6) |
Berihun et al. [58] | Polysect® | Acetamiprid | Ni (50) | FD | 46 | 18 | 77 | Cr | Arsenic acid | H3AsO4 | |
| FAO limit | 3000 | 3000 | 1200 | 1500 | 24 | - | 1508900 | 4.0As | ||||||||||
| Chicken | ||||||||||||||||||
| 0.10 | 0.20 | MAX | ||||||||||||||||
| 3 | ||||||||||||||||||
| Fungicides | Defarge et al., [38] | 578 | 314 | 33 | 4.1 | 251 | 0.5 | 23 | 39 | |||||||||
| Cu | 4300 | 1500 | 1500 | 75 | Copper acetoarsenite | C4H6As6Cu4O16 | As, Cu | |||||||||||
| MIN | 166 | 18 | 3.0 | 0.03 | 4.0 | 0.02 | 0.05 | 5.2 | Copper oxide | ®Cu2O | Cu | |||||||
| Folpet | As (260), Cr (2000), Ni (1200) | |||||||||||||||||
| FD | 3.5 | 17 | 11 | 137 | 63 | 25 | 460 | 7.5 | ||||||||||
| Duck | MAX | 682 | 199 | 41 | 2.5 | 64 | 0.07 | 6.8 | 16 | |||||||||
| MIN | 98 |
Table 7.
HM concentrations in irrigation water reported in some studies as compared to FAO approved maximum permissible limits.
| Elements | Cr | Cu | Zn | As | Cd | Pb | Ni | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg L−1 | |||||||||||||||||
| Max level (FD) * |
2.13 (21.3) |
4.62 | |||||||||||||||
| 2.00 | |||||||||||||||||
| 0.10 | |||||||||||||||||
| 35 | |||||||||||||||||
| 4.5 | |||||||||||||||||
| 0.3 | 7.0 | 0.03 | 0.01 | 8.4 | |||||||||||||
| 0.01 | 5.00 | 0.20 | Pb | 840 | 300 | 300 | 15 | Copper carbonate | CH2Cu | FD | 7.0 | 5.7 | 9.1 | 8.3 | 9.1 | 2.3 | |
| Hg | 57 | 17 | 17 | 0.9 | |||||||||||||
| Ni | 2O | Cu | |||||||||||||||
| 420 | 420 | 420 | 21 | Copper naphthenate | C22H14CuO4 | Cu | |||||||||||
| Se | 100 | 100 | 36 | 5.0 | Lead arsenate | 680 | C8F17LiO3S | Li | |||||||||
| 1.9 | |||||||||||||||||
| Poultry | MAX | 682 | Sodium meta-arsenite | NaAsO2 | As | ||||||||||||
| 314 | |||||||||||||||||
| Zn | 41 | 4.1 | 251 | 0.5 | 23 | 39 | Fungicide | ||||||||||
| Copper oxide | |||||||||||||||||
| 7500 | CuO | Cu | |||||||||||||||
| Copper bis(3-phenylsalicylate) | C26H18CuO6 | Cu | |||||||||||||||
| Copper abietate | C40H58CuO4 | Cu | |||||||||||||||
| Copper acetate | Cu2(CH3COO)4 | Cu | |||||||||||||||
| Copper carbonate | CH2Cu2 | ||||||||||||||||
| ) | |||||||||||||||||
| 2 | |||||||||||||||||
| Maronee® | Tebuconazole | As (90), Co (50), Cr (100) | |||||||||||||||
| Opus® | Epoxiconazole | Cr (90), Ni (60) | |||||||||||||||
| Pictor® | AsHO4Pb | BoscalidAs, Pb | |||||||||||||||
| As (300), Co (275), Cr (1000), Ni (600) | 2800 | 2800 | Lithium perfluorooctane sulfonate | O | |||||||||||||
| Teldor® | Fenhexamid | Cu | |||||||||||||||
| As (575), Cr (800), Ni (800) | |||||||||||||||||
| Herbicides | Defarge et al. | MIN[ | 77 | 15 | 2.0 | 0.03 | 2.5 | 0.02 | 0.01 | 5.2 | |||||||
| FD | 8.9 | 21 | 21 | 137 | 100 | 25 | 2300 | 7.5 | Copper chloride | ||||||||
| Cattle | CuCl2 | Cu | |||||||||||||||
| 38 | Copper hydroxide | H2O2Cu | Cu | ||||||||||||||
| Copper naphthenate | C22H14CuO4 | Cu | |||||||||||||||
| Copper oxychloride | (ClCu2H3O3)2 | Cu | |||||||||||||||
| Copper sulphate | AsO(OH) | As | |||||||||||||||
| ] | |||||||||||||||||
| R 3+® | Glyphosate-based formulations | As (375), Co (50), Cr (175), Ni (20) | CuSO | Rodenticides | |||||||||||||
| Eyetak® | MAX | 816 | 174 | 32 | |||||||||||||
| Prochloraz | As (200), Co (90), Cr (200), Ni (190), Pb (12) | ||||||||||||||||
| R Bioforce® | As (260), Cr (200), Ni (120) | ||||||||||||||||
| 3.4 | 79 | 0.6 | 6.3 | 19 | R Express® | As (60) | |||||||||||
| R GT+® | As (450), Co (150), Cr (100), Ni (50), Pb (10) | 4-Ca(OH)2 | Cu | ||||||||||||||
| Mercuric oxide | HgO | Hg | |||||||||||||||
| 140 | MIN | 49 | 12 | 1.6 | 0.04 | 0.8 | 0.02 | 0.01 | 4.2 | ||||||||
| FD | 17 | 15 | 20 | 85 | 99 | 30 | 630 | 4.5 | |||||||||
| Sheep | MAX | 431 | 215 | 20 | 1.4 | 22 | 2.4 | 2.6 | Mercurous chloride | Hg2Cl2 | Hg | ||||||
| Methoxyethylmercury chloride | C3H7ClHgO | Hg | |||||||||||||||
| Methoxyethylmercury acetate | |||||||||||||||||
| R WeatherMax® | As (500), Cr (100), Ni (20), Pb (10) | 12 | C5Barium carbonate | BaCO | |||||||||||||
| Bayer GC® | |||||||||||||||||
| 3 | Ba | ||||||||||||||||
| As (75), Co (60), Cr, (110) Ni (20) | |||||||||||||||||
| MIN | 42 | 8.4 | 1.7 | 0.3 | 8.0 | Clinic EV® | As (400), Co (90), Cr (150), Ni (20) | ||||||||||
| 0.2 | Glyfos® | As (200), Cr (1100), Ni (50), Pb (30) | H10HgOZinc phosphide | Zn3P2 | Zn | ||||||||||||
| Thallium sulfate | Tl2SO4 | Tl | |||||||||||||||
| Defoliants | |||||||||||||||||
| Glyphogan® | As (320), Co (125), Cr (100), Ni (40) | ||||||||||||||||
| Pavaprop-G® | Cr (110), N (190) | ||||||||||||||||
| Radical Tech+® | As (270), Co (70), Cr (50), Ni (50) | ||||||||||||||||
| Lonpar® | 2,4-D | As (160), Cr (150), Ni (180) | |||||||||||||||
| Matin® | Isoproturon | As (100), Cr (100), Ni (30), Pb (25) | |||||||||||||||
| Starane® | Fluoroxypyr | As (75), Cr (250), Ni (100), Pb (100) | 3 | Hg | |||||||||||||
| Phenyl mercuric acetate | C8H8O2Hg | Hg | |||||||||||||||
| Phenylmercury chloride | C6H5ClHg | Hg | |||||||||||||||
| Phenylmercury nitrate | C6H5HgNO3 | Hg | |||||||||||||||
| Sodium arsenite | NaAsO2 | As | |||||||||||||||
| Zinc borate | ZnB3O4(OH)3 | Zn, B | |||||||||||||||
| Zinc oxide | ZnO | Zn | |||||||||||||||
| Zineb | C4H6N2S4Zn | Zn | |||||||||||||||
| Herbicides | |||||||||||||||||
| Arsenic acid | H3AsO4 | As | |||||||||||||||
| Calcium arsenate | As2Ca3O8 | As | |||||||||||||||
| Sodium arsenite | NaAsO2 | As | |||||||||||||||
| Cacodylic acid | |||||||||||||||||
| Sodium dichromate | Na2Cr2O7 | Cr | |||||||||||||||
| Zinc chloride | ZnCl2 | Zn | |||||||||||||||
| Mercuric chloride | |||||||||||||||||
2.5. Land Irrigation
The irrigation of agricultural lands with contaminated water from surface water bodies as well as groundwater sources is another route of HM contamination in agricultural soil. The above irrigation practices are more frequently followed in some developing countries [9][15][16][* Numbers in parentheses represent concentration FDs, calculated based on average concentration obtained divided by FAO limits.
The causes for HM contamination in surface water bodies are both natural and anthropogenic. The natural causes include, among others, atmospheric deposition, geological and biological weathering, and climatic change. The anthropogenic causes include, but are not limited to, discharge of HM-contaminated agricultural, municipal, domestic, and industrial wastes. HM elements such as Pb, Ni, Cr, Cd, As, Hg, Zn, Cu, and others from diverse sources are transported to surface water bodies and irrigation canals through runoff, and to underground aquifers through vertical leaching with percolating rainwater [16][59][60][61][16,59,60,61]. Thus, irrigation of croplands using HM-contaminated water not only affects the growth and productivity of crops [62], but can also threaten soil quality. It should, however, be noted that the extent of crop damage will depend on the pH of the irrigation water, redox potential, and water solubility of the contaminated HM elements.
