Mg-based materials, from a comprehensive consideration of energy storage performance, raw material reserves, and prices, have demonstrated potential industrial applications as large-scale hydrogen storage materials. Nevertheless, Mg-based materials also have obvious disadvantages: as a hydrogen storage material, the hydrogen absorption/desorption rate is insufficient, as well as the high hydrogen absorption/desorption temperatures; as the electrode material of Ni-MH batteries, the reactions of Mg with alkaline electrolyte and corrosion are the main problems for applications.
1. Introduction
Energy is one of the most critical material resources for the development of human society. In recent decades, rapid population growth and heavy industry development have led to a significant increase in demand for energy. The traditional energy structure dominated by fossil fuels is no longer sufficient to support the sustainable development of human society. Therefore, one of the biggest challenges for today’s society is the search for sustainable, low-cost, clean energy sources. Furthermore, it is essential to achieve higher energy efficiency while gradually adapting new energy systems. In the future, all energy systems must use traditional energy more efficiently and gradually increase the use of renewable energy sources. Although natural resources such as solar, wind, geothermal, and tidal energy are renewable, their dependence on the environment and time zones leads to shortcomings, such as intermittency, an unstable capacity, and unpredictability
[1][2][3][4][1,2,3,4]. As the cleanest energy source, hydrogen energy is expected to transform the main carbon-based economy of modern society into a less- or even zero-carbon-based economy
[5][6][5,6]. However, as the lightest gaseous energy source, safe and efficient storage and transportation also limit the speed of the development and broad application of hydrogen energy
[7][8][9][7,8,9]. However, for the realization of energy structure adjustment and the efficient use of renewable energy, the problem of how to store energy safely and efficiently must be addressed and solved.
Energy storage is an essential intermediate link to achieving multi-purpose, easy-to-manage, and the efficient use of energy. At present, common energy storage systems include thermal, mechanical, electromagnetic, hydrogen, and electrochemical energy storage systems
[10][11][12][10,11,12]. Hydrogen and electrochemical energy storage have attracted increasing research interest and widespread attention in recent years, as they are compared to traditional energy storage methods. The ability of an energy storage technology to achieve large-scale applications in modern industry and real life is also closely related to the research and development of relevant materials
[13][14][13,14]. Energy storage materials play a key role in the efficient and multifunctional application of energy and are of crucial importance for the development, storage, and utilization of clean energy, as well as the construction of energy infrastructure. There are many kinds of energy storage materials that are widely used in industries. In addition, the safety of energy storage is mainly related to the environment and the choice of energy storage materials. Due to its high storage capacity, cheap price, and abundant reserves, Mg-based materials are considered one of the key types of energy storage materials with great potential applications
[15][16][17][15,16,17].
Nowadays, the research of Mg-based materials for energy storage applications mainly focuses on two areas: (1) as a storage medium for hydrogen in solid-state hydrogen storage tanks and (2) as an electrode material for Ni-MH batteries. However, both of these applications suffer from certain shortcomings. The former has a high thermodynamic stability resulting in high operation temperatures and poor kinetic performances, which severely limits the development and widespread use of Mg-based hydrogen storage materials
[18][19][18,19]. In the case of Ni-MH batteries, the performance largely depends on the rate of charge transfer on the electrode surface, which also includes the transfer rate of hydrogen between adsorbed and adsorbate states, as well as the diffusion of hydrogen in the adsorbed state between the interior of the material and the electrode surface
[20][21][22][20,21,22]. Moreover, the alkaline electrolyte in Ni-MH batteries also causes interfacial reactions with the electrode material, accelerating the corrosion of the electrode and, thus, affecting the electrochemical performance of the battery
[23][24][23,24]. Thus, the poor cycling stability performance and corrosion resistance of Mg-based materials are the obstacles for their application in Ni-MH batteries
[25][26][27][28][25,26,27,28]. More and more research results show that the performance of Mg-based materials, either as hydrogen storage media or electrode materials, is closely related to the surface structure and surface state.
[29][30][31][32][29,30,31,32].
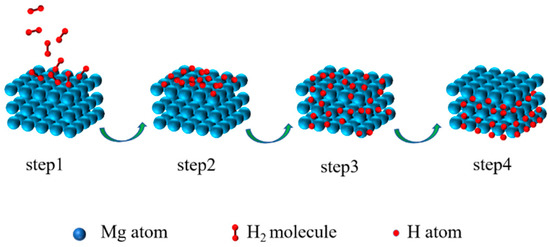
On the one hand, as a hydrogen storage material, the surface layer of Mg-based materials dramatically impacts performance. It directly affects the physical and chemical adsorption and the decomposition of H
2 on the material surface, as well as the surface permeation rate of H. These factors ultimately affect the kinetic performance of hydrogen adsorption and desorption. The hydrogen absorption reaction process of materials can be described as follows (
Figure 1)
[33]: step 1: H
2 is physically adsorbed on the surface of particles by van der Waals forces; step 2: H
2 is chemisorbed and dissociated to H on the surface of particles; step 3: H penetrates the lattice through the surface and diffuses to the interior of the alloy; step 4: the alloy phase transforms to the hydride phase. The hydrogen desorption reaction can be considered the reverse process of the hydrogen absorption reaction
[34]. The dissociation energy barrier of H
2 on the Mg surface is as high as 218 kJ mol
−1 H
2 [35]. The slow surface permeation rate of H on the material surface is also a key factor affecting the hydrogen absorption reaction kinetics of Mg-based materials. As is known, Mg-based materials are extremely prone to oxidation during the preparation process. The oxide layer formed on the surface hinders the decomposition of H
2 and the surface permeation of H, leading to a significant decrease in the hydrogen absorption/desorption rate
[36]. Moreover, prolonged hydrogen absorption/desorption can also lead to the pulverization of the particles and the poisoning of impurity gas, which reduces the hydrogen storage capacity of the material and shortens its service life. Thus, in order to enhance the hydrogen absorption kinetics, it is necessary to increase the rate of decomposition of H
2 on the material surface, the permeation rate of H on the surface, and the ability of the surface to resist impurity gas poisoning. However, these all directly depend on the composition, structure, and state of the material surface.
Figure 1. Schematic illustration of the hydrogen absorption process of Mg.
On the other hand, when Mg-based materials are prepared as electrodes for Ni-MH batteries, the main processes that occur at the electrodes during charging are the decomposition of water into H at the material’s surface and the diffusion of H to the surface and inside the material. When the counter-current is applied, H is released and oxidized to water
[37][38][37,38]. Two main factors dictate the electrochemical kinetic performance of electrodes: the ability of hydrogen diffusion inside the material and the ability of charge transfer on the surface of the material
[39][40][39,40]. Furthermore, electrons are conducted to the electrolyte through the propagation medium of the material surface, so a key factor influencing the electrode reaction is a surface with good electron conductivity. In addition, the surface of the electrode material is also highly susceptible to corrosion in alkaline electrolytes. As a result, the material surface’s ability to resist corrosion also plays a crucial role in determining the electrode’s cycling stability and service life.
2. Surface Modification of Magnesium-Based Materials
The properties of Mg-based materials are closely related to their composition, crystal structure, and surface state. Generally, thermodynamic properties, such as the maximum capacity and enthalpy change, are directly related to the material composition and crystal structure
[41]. However, the surface state significantly impacts the kinetics
[42], as well as the properties of surface corrosion resistance, impurity toxicity resistance, and electrocatalytic activity. These factors, in turn, affect the overall performance of the material. To improve the performance of Mg-based materials, various surface modification techniques, such as surface coatings, surface catalysis, nanocrystallization and amorphization, and the formation of core–shell structures, are often employed. These techniques have supported the application of Mg-based materials in fields such as hydrogen storage and Ni-MH batteries.
2.1. Surface Modifications of Hydrogen Storage Material
In recent years, an important research direction for Mg-based materials is their application as a hydrogen storage and transportation medium. In this field, the hydrogen absorption/desorption rate, temperature, and storage capacity are the primary concerns. From a kinetic perspective, the main reasons for the slow hydrogen absorption reaction of Mg-based materials include the following: (1) the MgO or Mg(OH)
2 layer on the material’s surface inhibits the decomposition of H
2 and the penetration and diffusion of H on the surface
[43], and (2) after the initial production of MgH
2 on the surface, the subsequent diffusion of H gradually becomes more difficult
[44][45][46][44,45,46]. Therefore, it is essential to accelerate H
2 decomposition and H diffusion on the material surface to improve the hydrogen absorption/desorption rate. Surface modification techniques, such as surface catalytic treatment, nanocrystalline treatment, and the formation of core–shell structures, are crucial for enhancing the surface performance of Mg-based materials.
2.2. Surface Modifications of Electrode Materials in Nickel–Metal Hydride Batteries
Ni-MH batteries are secondary new energy batteries with Ni(OH)
2 as the positive electrode, metal hydride (MH) as the negative electrode, and an alkaline solution as the electrolyte
[47][48][108,109]. Ni-MH batteries have been widely used in commercial hybrid vehicles, such as Toyota, Honda, and Ford, since 1999 due to their high level of safety, environmental friendliness, and good low-temperature properties
[49][50][110,111]. However, the capacity, durability, and discharge performance (kinetics) of the battery depend primarily on the inherent properties of the electrode materials, especially for the preparation of active materials for the negative electrode
[51][52][112,113]. There have been a series of studies on negative electrode materials for Ni-MH batteries, such as AB
5, AB
2, AB
3, AB, A
2B, and Mg-based materials
[53][54][55][56][57][114,115,116,117,118]. However, with these materials for Ni-MH battery applications in the field of high power, there are still some problems, such as easy polarization, a poor discharge ability at a high rate, easy corrosion in alkaline electrolytes, and poor cycling stability.
The performance of Ni-MH batteries depends not only on the bulk structure of the electrode but also on its surface state. Mg-based alloys are widely used as the negative electrode in Ni-MH batteries due to their exceptional electrochemical properties, cost-effectiveness, and abundant reserves of raw materials
[47][108]. However, the appearance and growth of its MgO/Mg(OH)
2 surface layer impede electron transport at the electrode/electrolyte interface and create a large discharge potential, resulting in a lower discharge capacity and a poorer kinetic performance of the electrode material
[58][119]. Moreover, the interfacial reaction between the electrode material and the electrolyte can seriously affect the electrochemical performance of the batteries
[59][60][120,121]. The optimization of the performance of Mg-based electrode materials and Ni-MH batteries can be achieved through surface treatment. Common methods of modification include surface coating, nanocrystallization and amorphization, and surface catalysis.
2.3. Analysis of the Application of Magnesium-Based Materials in Existing Fields
The research and development of energy storage materials are crucial to the utilization and storage of energy. The ideal energy storage materials for a wide range of industrial and residential should consider factors such as the raw material and preparation costs, energy storage capacity, operating conditions, and service life. Mg has abundant reserves (about 2%
[61][157]) and good hydrogen storage properties in the Earth’s crust. Currently, the research on the performance of Mg-based materials in hydrogen storage mainly focuses on solid-state hydrogen storage and Ni-MH batteries. Although researchers have shown that Mg-based materials show relatively excellent performance in hydrogen storage and electrochemical energy storage, which still not enough to achieve large-scale adoption.
In accordance with what is described in this paper, Table 1 and
Table 2 briefly summarize the relevant properties of some modified Mg-based materials in the field of hydrogen storage and Ni-MH batteries, respectively.
Table 1. Partial hydrogen storage properties of Mg-based materials after surface modification.
| Ref. |
| Nd0.7Mg0.3Ni3 |
Coating layer (Ni) |
355 |
79.6 (C200) |
202.6 |
[75][131] |
| Nd0.7Mg0.3Ni3 |
Coating layer (Co) |
337 |
76.5 (C200) |
163.6 |
[75][131] |
| La0.8Mg0.2Ni3.4Al0.1 |
Coating layer (polyaniline) |
391.8 |
87.5 (C100) |
98.9 |
[76][129] |
| Mg2Ni |
Coating layer (rGO) |
594 |
60 (C50) |
225.9 |
[77][132] |
| Mg2Ni |
Coating layer (Ni) |
946 |
38 (C10) |
91.6 |
[78][128] |
| Mg0.9Ti0.1Ni |
- |
455 |
31.6 (C20) |
- |
[79][139] |
| Mg0.8Ti0.1Pd0.1Ni |
Amorphous structure |
326.5 |
74.2 (C20) |
184 |
[79][139] |
|
La9Ce1Mg80Ni5
(Ni + 3 wt% GR) |
Nanocrystalline and amorphous structures (ball milling 20 h)
|
315.4 |
73 (C20) |
- |
[80][140] |
|
La9Ce1Mg80Ni5
(Ni + 3 wt% GR) |
Nanocrystalline and amorphous structures (ball milling 80 h) |
344.9 |
55 (C20) |
- |
[80][140] |
|
La0.88Mg0.12Ni2.95Mn0.10Co0.55Al0.10
|
Surface catalytic phase
(Ni-Cu-P) |
361 |
84.8 (C200) |
378.9 |
[81][133] |
|
La0.75Mg0.25Ni3.2Co0.2Al0.1
|
Surface catalytic phase
(Cu-Pd) |
386.3 |
82.7 (C100) |
483.8 |
[28] |
|
La1.7Mg1.3Ni9
|
Surface catalytic phase (Y2O3, milled 10 h) |
392.2 |
90 (C30) |
- |
[82][151] |
|
La1.7Mg1.3Ni9
|
Surface catalytic phase (Y2O3, milled 20 h) |
387 |
95 (C30) |
- |
[82][151] |