2. Background of FGFs in the Development of Nervous System
FGFs, a broad family of polypeptide growth factors, were originally isolated and purified from the pituitary gland of bovines
[13][14]. Many studies have discovered that FGFs have a variety of roles in the development of both vertebrates and invertebrates, and are extensively expressed in almost all cell types
[14][15][16][17][15,16,17,18]. FGFs comprise approximately 200 amino acids, with a highly homologous core region of 120 amino acids
[18][19]. The core region contains the binding site, which has an affinity for heparan sulfate proteoglycans (HPSGs) on cell surfaces or in the extracellular matrix
[18][19]. Then, N and C sequences on the side of the core region enrich the diversity of FGF family members
[19][20]. Acidic FGF (aFGF) and basic FGF (bFGF), commonly known as FGF1 and FGF2, are the first two FGFs isolated, purified and sequenced
[20][21][21,22]. To date, 23 FGF members have been identified in different species. Through phylogenetic analysis, these FGFs are typically divided into seven subfamilies: FGF1, FGF4, FGF7, FGF8, FGF9, FGF11 and FGF15/19
[11]. While FGF15 in rodents is orthologous to FGF19 in other vertebrates, only 22 FGF family members can be found in these species when it comes to the FGF15/19 subfamily. Hence, not every species contains all FGF subtypes. Some studies have also proposed that there are eight FGF subfamilies, in which FGF3 is taken out of the FGF7 subfamily as a single subfamily with only one member. Generally, FGFs are divided into three groups according to their mechanisms of action: paracrine FGFs, endocrine FGFs and intracellular FGFs. Most FGFs are secreted outside the cells and bind to FGFRs to exert their effects. They usually act as autocrine, paracrine or juxtacrine factors, which are called canonical FGFs, except for three “hormone-like” FGF15/19 subfamily members (FGF15/19, FGF21 and FGF23) acting as endocrine factors
[10]. Additionally, four intracellular members of the FGF11 subfamily (FGF11, FGF12, FGF13 and FGF14) are referred to as FGF homologous factors (FHFs) for not binding with FGFR, which are crucial controllers of cardiac and neural excitability.
Multiple studies have highlighted the importance of FGF expression in the nervous system as development progresses
[10][22][10,23]. Although FGFs are widely secreted in almost every tissue and organ, the organizing centers of FGFs in the developing central nervous system are well-reported. For instance, FGF8, derived from the midbrain-hindbrain boundary (MHB), commonly known as the isthmic organizer (IsO), plays an instrumental role in assigning midbrain and hindbrain fates
[23][24][25][26][27][28][29][24,25,26,27,28,29,30]. Moreover, FGF signaling in IsO functions does not appear to be limited to the orderly production of certain neuronal population types. In addition to mediating fate mapping activities, IsO FGF8 regulates the pathway finding of various axons at later stages in development, either in a direct manner, such as in trochlear motor axons, or in an indirect manner, such as in retinal ganglion cells (RGCs) axons, and also in repelling dopaminergic axons
[30][31][32][33][31,32,33,34].
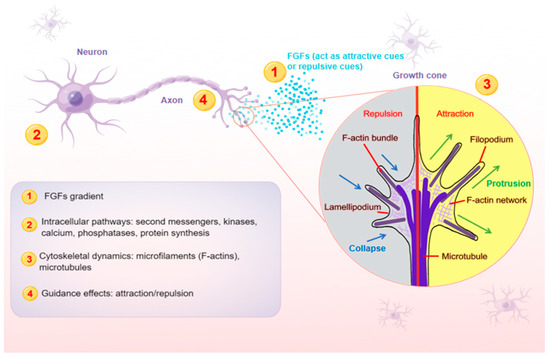
In the nervous system, axons play an essential role in conducting electrical impulses (action potentials) from the body of neurons to their targets, such as muscles, neurons and glands. Therefore, it is crucial for neurons to stretch their axons along the correct paths to reach specific targets among myriad cells. To provide directional information for growing axons, the expressions of FGFs need to be restricted within a certain range of action, and then form a concentration gradient (
Figure 1), exerting either attractive or repulsive effects in the proper axonal pathway
[34][35]. As FGFs are secreted, they can bind to cofactors, such as HSPG cofactors or Klotho coreceptors, which are especially important for endocrine FGFs, to spatially limit their effects. HSPG cofactors control the diffusion and availability of secreted FGFs, regulating the effectiveness of axon guidance. FGFs are able to diffuse for some distance before becoming bound, which is thought to produce shallow gradients or provide long-range guidance signals. For example, due to their poor affinity for HSPGs, endocrine FGFs can diffuse into the circulation from the place of synthesis. In contrast, FGFs that are tethered right away after release tend to produce steep gradients that provide short-range guidance information. The interaction of FGFs with HSPGs in the extracellular matrix results in creating FGFs reservoirs and forming FGFs gradients, both of which are required for paracrine signaling
[35][36].
Figure 1. A schematic model illustrating the main steps (1–4) at which FGFs guide axons. Axon is a long and slender projection of a neuron, which is also defined as a nerve fiber. Axon guidance is an essential process for neural circuit formation. Growth cones at the tips of axons are crucial for detecting the guidance cues such as FGFs. Multiple guidance instructions then activate cellular responses, which recruit and assemble intracellular signaling components. By integrating diverse intracellular pathways, growth cones make corresponding cytoskeletal changes. Axons can thus exert appropriate motile actions.
3. Effects of FGFs on the Axon Guidance
FGFs within the developing neural system are necessarily required for patterning, neurogenesis and maintaining the physiology and homeostasis of neurons
[36][37][37,38]. In addition to these fundamental activities, many studies have shown that FGFs, at the level of cells, act as guidance cues in the later development stages. It is obvious that FGFs can guide different axons to grow along their proper trajectory in various ways (
Table 1).
Table 1.
Guidance effects of FGFs on axons in vertebrates.
3.1. Direct Axon Guidance Effects of FGFs in Vertebrates
Irving et al. found that FGF8 can make space for cerebellar development by inhibiting expressions of Hox genes in rhombomere 1, the anterior segment of the vertebrate hindbrain, which will develop into the cerebellum
[47][48]. Inside this rhombomere within and posterior to the IsO, the trochlear motor neurons that innervated the eye muscle developed only in this restricted FGF8-positive area
[47][48]. Studies on the formation of rhombomeres provide additional support for the involvement of FGF signaling in axon guidance by demonstrating how FGFs in the cerebellum function as synaptogenesis regulators and trochlear motor axon attractants
[30][48][31,49]. In 2002, Irving and his partners showed that isthmic-derived FGF8 was also employed to direct trochlear axons out of the neural tube in chick embryos. They initially demonstrated how trochlear axons were drawn to FGF8-soaked beads in vitro, and how these beads implanted in vivo redirected the growth of those axons
[30][31]. Before leaving the neural tube, FGF8 and isthmic tissue attracted the trochlear motor axons in the rostral hindbrain to turn dorsally and away from the floor plate of the isthmus, even though they could not significantly promote the growth of these axons. These axons’ trajectory is probably constrained along the segmental boundary of the MHB by FGF8 secreted along their route. Compared with many other classical chemoattractants, such as netrins, hepatocyte growth factor (HGF), and neurotrophins, FGFs exert a slightly distinct effect on trochlear axons because they cannot promote neurite outgrowth
[49][50][51][50,51,52]. Furthermore, trochlear axons deviated from their correct pathway in explant cultures of the entire midbrain–hindbrain boundary area treated with the FGF8 inhibitor, FGFR blockers, or other defasciculation factors
[52][53]. All these findings suggest that isthmic-derived FGF8 attracts trochlear axons, and plays a positive role in the formation of the IV cranial nerves.
In addition to the studies on trochlear motor axons, extensive research has shown the guidance roles of FGFs on other axons. For example, during the development of the cerebral cortex, FGF2 can elicit cortical pyramidal neurons’ interstitial axonal branching, leading to the collateral branching of layer five projection neurons extending to the destination
[53][54]. Moreover, the dermomyotome expressed FGF8 when medial-class spinal motor neuron (MMCm) axons navigated toward this targeted interembryonic development
[24][54][25,55]. Indeed, studies have demonstrated that FGF2, FGF4, and FGF9 were secreted in the dermomyotome at the moment of MMCm axonal pathway finding, suggesting that FGFs serve as important components guiding spinal motor neurons
[55][56][57][56,57,58]. Finally, according to the results of Shirasaki’s research, FGF2, FGF4, FGF8, and FGF9 act as the axonal chemoattractants and the neurotrophins on MMCm axons in vitro
[38][39][39,40]. Additionally, at early developmental stages, anosmin-1 encoded by the Kallmann syndrome gene (KAL-1) was essential for the differentiation and maturation of olfactory ensheathing cells (OEC) via the FGF2 signaling pathway, which could be blocked by the FGFR inhibitor SU5402
[58][59]. Then, at later embryonic and post-natal development, olfactory sensory axons crossed the nervous system boundary and targeted the olfactory bulb (OB). This pathway-finding process depends on the proper glial environment produced by OEC wrapping the olfactory sensory axons. Studies have demonstrated that FGF1 expressed by OEC regulates the olfactory sensory axon growth between the olfactory neuroepithelium and the OB, which suggests that FGF1 is essential in forming the olfactory pathway
[59][60].
3.2. Indirect Axon Guidance Effects of FGFs in Vertebrates
Recently, many data points suggest that FGFs can also guide the pathway finding of axons indirectly by patterning other guidance cues. Retinal ganglion cell (RGC) axons migrate from the eye to their primary contralateral destination in the brain, the optic tectum, during the development of the visual system. Additionally, in the posterior optic tectum at the IsO, several secreted FGFs act as guidance cues. Many previous pieces of research have proved that FGF2 regulates the growth and guidance of RGC axons at later stages of development in vivo
[60][61][62][61,62,63]. Webber’s laboratory has previously confirmed that RGC axons were directly repelled by FGF2 both in vivo and in vitro
[45][46]. Consistently, Song et al. have also found that FGFs acted as repellents to guide RGC axons away from the mid-diencephalon and towards the optic tectum
[63][64]. Whereas, while RGC axons were repelled by FGF8 ectopically injected into the axonal trajectory in vivo, this repulsive response was not observed in vitro. It was possible that FGF8 indirectly directed RGC axons in vivo by inducing neuroepithelial cells to secrete a component that repelled these axons
[58][59]. It has been demonstrated that FGF8 controlled the synthesis of the transcription factor engrailed-2, which was generated in the mesencephalon along a decreasing gradient from caudal to rostral
[64][65]. Under the positive regulation of engrailed-2, the two Eph receptor tyrosine kinase ligands (ELF-1 and RAGS) were likewise expressed in a decreasing caudal-to-rostral gradient throughout the optic tectum and then controlled the patterning of retinal axon terminals
[65][66]. Another compelling case is that FGF8 can indirectly repel the axons of midbrain dopaminergic neurons (mDAN) extending through the diencephalon. How FGF8 regulates the rostro-caudal growth polarity of mDAN axons has been proven by inducing the expression of the chemorepellent semaphorin 3F (sema3F) in the midbrain
[44][45]. Moreover, in the previous in vitro study, FGF10 enhanced the outgrowth of SAG neurites and maintained these neurons’ survival, which could not be inhibited by SU5402. Therefore, additional signaling pathways in this development process may be indirectly activated by FGF10
[40][41]. Furthermore, FGF3 and FGF8-dependent FGF22 signaling has been found to indirectly change the cytoskeleton during axon guidance and shape the development of the midbrain, which may act by regulating WNT1
[66][67][67,68]. Additionally, the indirect guidance method may explain the results that, despite lacking FGFR1 signaling, some transplanted ES cell-derived MMCm motoneurons appropriately projected to epaxial muscles
[68][69]. Overall, these indirect guidance fashions significantly contribute to the variety and complexity of FGFs’ activities in axonal pathfinding and the subsequent development of the nervous system.
3.3. FGF’s Axon Guidance Functions in Invertebrates
FGF signaling is also proven to participate in the invertebrate nervous system, including the axonal guidance process. FGF2 expressed in the head of the cockroach embryos in vivo served as a crucial antagonist of an axon growth inhibitor generated in the thorax, which was essential in guiding the pioneer axonal pathway to turn and elongate proximally in the coxa or into the CNS
[17][18]. In addition, FGF signaling indirectly guided the proper extension of axons and maintained the correct position of different classifications of axons in
Caenorhabditis elegans [69][70]. Furthermore, the heartless, which is the fly homolog of the vertebrate FGFR, is required for the outgrowth of axons in cultured Drosophila neurons
[70][71]. Nonetheless, the roles of FGFs in the axonal pathway-finding process in invertebrates have not been extensively explored.
4. Concentration-Dependent Responses of Growth Cones to FGFs
4.1. Continuous Changed Responses of the Same Axons over Time and Space
At different developmental stages, the surrounding and internal environments of the same axons undergo continuous changes, which lead to more complex guidance effects of FGFs. Many researchers found that FGFs may have a dose-dependent dual function during the axon guidance process (
Table 1). The patterning function of FGFs begins at very early stages of development, so it is reasonable to speculate that the remaining established gradients of FGFs play a significant role in establishing the complicated neuronal connectivity of the nervous system. Previous research has found that the misrouting of thalamocortical axons (TCAs) could result from early ectopic sources of FGF8
[71][72]. The guidance of TCAs is also directed by other FGFs, which may exert bifunctional guidance roles. For example, the low concentration of FGF10 and FGF3 attracts TCAs; in contrast, high levels of FGF3 and FGF10 in the hypothalamus can serve as a chemorepellent, directing TCAs into the ventral telencephalon and away from the hypothalamus
[42][72][43,73]. Furthermore, a previous study demonstrated that FGF3 and FGF10, the FGF7 subfamily members, can not only regulate the differentiation of midline-derived progenitor cells during the hypothalamic infundibular development, but also guide the hypothalamic axons to the median eminence in the later stage of hypothalamic development bifunctionally
[43][73][44,74]. The research showed that low-concentration FGF3 and FGF10 attracted hypothalamo-neurohypophyseal (H-NH) axons, while high-concentration FGF3 and FGF10 repelled H-NH axons
[43][44]. These studies demonstrate that FGF3 and FGF10 exert concentration-dependent effects on the diencephalic axon guidance, helping axons navigate to their appropriate destinations. In the study by Kristen et al., they found that only low-concentration FGF8, FGF10, and FGF19 could significantly promote chicken statoacoustic ganglion (SAG) axon asymmetric outgrowth in vitro
[40][41]. Additionally, the axonal guidance effects of FGFs are not only concentration-dependent, but also time-specific. Previous studies have proved that FGF2 enhances the survival of chick SAG neurons in vitro, but only in the early (E2–3) and later (E8–16) stages
[74][75][75,76]. In contrast, in Kristen’s study, only E4 SAG axons did not respond to the bioactivity of FGF2
[40][41]. Thus, the responses of chick SAG to FGF2 may be time-sensitive
[75][76][76,77].
4.2. The Molecular Mechanism behind the Concentration-Dependent Responses
It is unnecessary to categorize any guidance cue as either attractive or repulsive since it behaves differently in different circumstances. Firstly, the response of an axon to a given guidance cue will be influenced by other crosstalk cues in the surrounding environment. At the tips of growing axons, growth cones are identified as the highly motile sensory apparatus that can respond to extrinsic guidance instructions and then guide axons to their specific targets
[52][53]. According to the embryological research concentrating on the topographic projections of spinal motor axons, distinct axon pathfinding of motor neuron subtypes differed in their intrinsic capabilities. The finding shows that motor axons have been genetically preprogrammed to detect guidance cues before they reach their targets
[77][78]. Hence, based on the diverse complement of expressed receptors, the same guidance cue can be interpreted variously by distinct neurons. Secondly, the responses of axons to the guidance cues need to be plastic over space and time, and much research has shown that intracellular signaling systems in neurons also critically affect the roles of guidance cues (
Figure 1). Cyclic nucleotide levels (cAMP and cGMP activity) and intracellular calcium concentration are demonstrated to be two key regulators participating in modulating the guidance responses
[5][78][79][80][81][5,79,80,81,82]. Elevation of intracellular calcium can convert repulsion into attraction
[81][82]. Moreover, the effect of the cAMP-dependent or cGMP-dependent pathways can be mediated by protein kinase A (PKA) or protein kinase G (PKG), which can modulate the synthesis of cytoskeleton-associated proteins
[82][83]. Many experiments have confirmed that reducing cAMP or cGMP levels switches attraction to repulsion, while increasing cAMP or cGMP favors converting a repulsive response to an attractive one
[41][79][81][83][42,80,82,84]. However, the level of cGMP in dendrites may be higher than in axons because the guanylyl cyclase is only detected in dendrites
[84][85]. Consistent with the mechanism of many guidance cues, studies suggested that cAMP and other regulating molecules were crucially important in the concentration-dependent responses of growth cones to FGFs over space and time
[41][79][84][42,80,85].