Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Parvaneh Sheydaei.
Tripleurospermum, a prominent genus within the family Asteraceae, is recognized for its therapeutic potential in treating various ailments, including skin, digestive, and respiratory diseases; cancer; muscular pain; and stress and as a sedative. Through extensive phytochemical studies regarding the Tripleurospermum species, numerous chemical compounds have been identified and classified into distinct classes, predominantly encompassing terpenes, hydrocarbons, steroids, hydrocarbons, oxygenated compounds, flavonoids, tannins, alcohols, acids, melatonin, and fragrant compounds.
- Tripleurospermum
- phytochemistry
- antioxidant activity
- antimicrobial activity
- cytotoxicity
1. Introduction
In recent years, there has been a growing trend in the utilization of natural medicines to treat illnesses due to their reduced adverse effects, cost-effectiveness, and wide availability [1]. Although several articles have already explored the medicinal potential of active compounds, namely active molecules, derived from the genus Tripleurospermum in various therapeutic areas [2,3,4,5,6][2][3][4][5][6], it is imperative to conduct further investigations into the therapeutic and toxicological properties of this plant genus. The aim of this review was to comprehensively analyze the botanical characteristics, traditional uses, phytochemistry, pharmacology, and toxicity profiles of different Tripleurospermum species. Moreover, wresearchers intended to facilitate and guide future research endeavors by documenting the ethnopharmacological applications of Tripleurospermum. Noteworthy scientific contributions have been made on diverse facets of the genus Tripleurospermum, such as its pharmacology [2], toxicity [2], biochemical properties, the pharmaceutical potential of proteins and peptides derived from this genus [7[7][8][9][10][11][12],8,9,10,11,12], as well as its chemical constituents [2,13,14][2][13][14]. Lastly, thires reviewerachers provides a concise summary and outlines future research directions in the field of the Tripleurospermum genus. In the subsequent sections, an overview of the taxonomy, stomatal characteristics, achene, as well as the anatomical features of leaves and stems observed in various species of Tripleurospermum is presented. The genus Tripleurospermum is a member of the tribe Anthemideae, in the family Asteraceae, comprising nearly 40 species. It is closely related to the genus Matricaria L. which consists of approximately seven well-defined taxa [15]. In the geographical region of Turkey, Tripleurospermum is reported to encompass 40 species and 32 taxa [3,16[3][16][17][18][19][20][21],17,18,19,20,21], of which 12 exhibit distinct leaf and achene/cypsela anatomical characteristics [22]. Recent taxonomic research by Ref. [23] identified Tripleurospermum eskilensis as a new species, thus expanding the number of Tripleurospermum taxa in Turkey to 33. Notably, Turkey has been recognized as the primary source of the cultivated variety of Tripleurospermum [24,25][24][25]. In Russia, the genus Tripleurospermum comprises 17 species [26], while Iran is home to 6 species of Tripleurospermum [13,27][13][27].
The taxonomic classification of the genus Tripleurospermum remains a subject of contention, particularly due to its species’ distinct characteristics. Initially, the genus Tripleurospermum was classified under the genus Matricaria L. but was later reclassified as a separate genus based on two distinguishing factors: the unique shape of its achene and the prevalence of tetrasporic embryo sacs [4]. However, it should be noted that Refs. [5,6][5][6] have erroneously reported Matricaria L. as part of the Tripleurospermum genus. Subsequent investigations confirmed the association of Tripleurospermum with Anthemis L. rather than with Matricaria L. [28]. In addition, limited chromosomal information is available for both Tripleurospermum and Anthemis L. The ploidy levels observed in Tripleurospermum range from 2n = 2x = 18 to 2n = 3x = 27 to 2n = 4x = 36. There are four ploidy levels (2x, 3x, 4x, and 5x) in all Tripleurospermum species [29]. In contrast, Matricaria L. was found to have a single ploidy level (2x) [8]. Polyploids have been proposed as a potential mechanism contributing to the diversification and divergence of the genus Tripleurospermum [3,25][3][25]. Furthermore, the outermost layer of this genus’ leaves has been identified as a significant factor in taxonomic differentiation and to play a role in the development of stomatal complexes, including those found in achenes [30,31][30][31]. It is worth noting that anatomical features such as stomatal length, vascular bundle size, and palisade sclerenchyma thickness are important for distinguishing between Tripleurospermum species and for establishing a correlation between ploidy level and anatomical structures across different Tripleurospermum species [22,32][22][32].
The stomata of Tripleurospermum species are distributed on both the upper and lower surfaces of their leaves, with equal stomatal density reported [22]. Stomatal characteristics such as stomatal frequency, guard cell length, and stomata plastids’ diversity have been used as morphological indicators for assessing ploidy levels in different plant species [33,34,35,36][33][34][35][36]. Studies have demonstrated that polyploid plants exhibit longer stomata than diploid plants [37,38][37][38]. Furthermore, a positive correlation was observed between ploidy level, stomatal size, and altitude in Tripleurospermum species [3]. Taxonomically, stomatal and vascular bundle sizes are associated with the degree of polyploidy, which holds significance in distinguishing different Tripleurospermum species, particularly within the Turkish endemic species [30].
In all species of the genus Tripleurospermum, achenes exhibit a general similarity, but their anatomical structure, i.e., their pericarp, seed coat, endosperm, and seed lobe, display distinct variations among species [22], which aligns with previous findings reported by Ref. [30]. The morphology and anatomy of achenes are known to contribute to the systematic and phylogenetic understanding of the Anthemideae tribe within the Asteraceae family [15,39,40,41,42,43,44][15][39][40][41][42][43][44]. Furthermore, achene morphology, such as size, shape, number of ribs, slime formation, and pericarp shape, are crucial in the classification of Tripleurospermum taxa [31,39,43,45][31][39][43][45].
The leaf anatomy of Tripleurospermum species bears similarities to that of Matricaria L. [22]. The leaf anatomy of Turkish Tripleurospermum taxa typically consists of lower and upper epidermis, parenchymatous mesophyll, and a vascular bundle [46]. The epidermal cells are isodiametric, resulting in nearly straight walls in both the lower and upper epidermis [30]. The leaf surfaces are covered with nonglandular uniseriate and multicellular trichomes, although T. corymbosum has been reported to be glabrous [22,30][22][30]. The mesophyll blades primarily comprise dorsiventral and palisade parenchyma cells, similar to the mesophyll structure observed in the Asteraceae family [22,47,48][22][47][48]. In addition, all taxa of Tripleurospermum exhibit either one major vascular bundle or two medium-sized vascular bundles with a secondary vascular bundle [22]. It is worth noting that the large vascular bundle, which constitutes 50% of the mesophyll’s area, holds taxonomic significance in the delimitation of the taxa [22,30,47][22][30][47].
Regarding stem anatomy, Tripleurospermum stems are usually rounded, but some species, such as T. caucasicum, T. melanolepis, T. rosellum var. album, T. parviflorum, T. sevanense, T. transcaucasicum, and T. monticola [32], were reported to have slightly ridged stems with seven to nine ribs [32]. The stem anatomy of the studied Tripleurospermum species, including M. chamomilla [49], T. baytopianum, T. caucasicum, T. monticola, and T. transcaucasicum [50[50][51],51], generally exhibits similarities. However, the stem anatomy of Matricaria L. and Tripleurospermum is considered of minor importance for taxa delimitation [32].
2. Habitat, Distribution, and Ecology
The Asteraceae family represents the largest and most prominent family of plants [52]. The related extensive research has focused on the remarkable diversity of the species and genera, global distribution, and effective plant species [42].
Tripleurospermum Sch. Bip., a genus within the family, has been cultivated in various temperate regions of Europe and Asia alongside other species found in north Africa and North America [12,15,20,22,25][12][15][20][22][25] (Figure 1). Species belonging to this genus are characterized as herbaceous, either annual or perennial in nature [14].
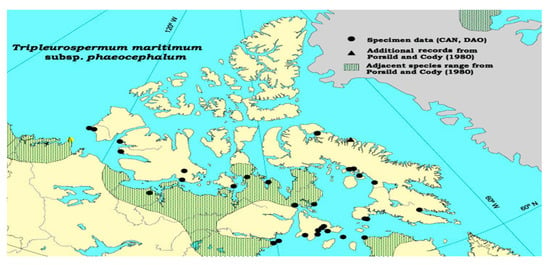
Figure 1. Distribution of the cytotype of Tripleurospermum maritimum in North America and the northern hemisphere. ● − 2n = 18 [53].
3. Phytochemical Compounds of the Genus Tripleurospermum
The essential oil composition of Tripleurospermum decipiens flowers has been previously investigated, and the main reported compounds are matricaria esters [44] (Figure 2). Matricaria esters have also been identified as compounds of T. inodorum [54] and T. disiforme [55] (Figure 2).
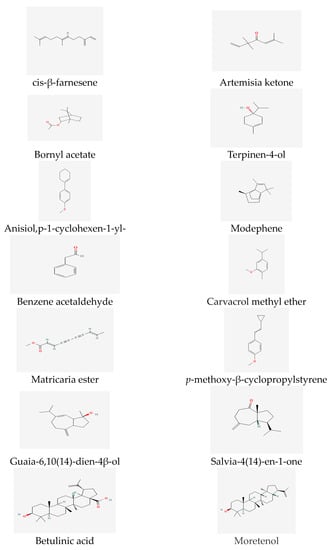
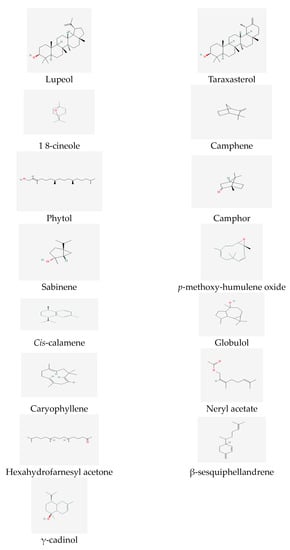
Figure 2. Essential oil compounds of Tripleurospermum spp.
The presence of terpenoids in Tripleurospermum decipiens was confirmed based on a reference study [56]. Furthermore, the primary compounds found in the essential oil of the aerial parts of Tripleurospermum disiforme include trans matricaria ester (39.93%), cis-calamenene (22.99%), (Z)-b-farnesene (12.54%), b-maaliene (7.98%) and b-sesquiphellandrene (2.22%) [57].
Previous reports have identified the significant essential oil compounds in the flowers, leaves, and stems of T. inodorum. In the flowers, important compounds include artemisia ketone (14.4%), terpinene-4-ol (5.5%), 1,8-cineole (5.1%), sabinene (4.7%), and tricosane (4.6%) [58] (Figure 2). The leaf is characterized by important compounds such as caryophyllene oxide (16.0%), phytol (12.1%), spathulenol (5.9%), hexahydrofarnesyl acetone (3.8%), and salvia-4(14)-en-1-one (3.5%), which exhibit relatively high percentages. The main compounds in the stem essential oil include neryl acetate (12.8%), (E)-β-farnesene (12.5%), phytol (12.1%), guaia-6,10(14)-dien-4β-ol (10.8%), γ-cadinol (7.8%), nonacosane (7.3%), decanoic acid (6.3%), and caryophyllene oxide (4.6%) (Figure 2).
Phytochemical research on T. insularum Inceer & Hayırlıoglu-Ayaz [19] has revealed that the basic compounds of the headspace and essential oils are fatty acids and n-alkanes (38.43–59.22%), including n-octacosane, linoleic acid, and n-hexacosane [59]. Additionally, sesquiterpenes (13.45%) and β-sesquiphellandrene (9.29%) were identified as the main compounds in the essential oil of the genus T. insularum Inceer & Hayırlıoglu-Ayaz [19]. Moreover, β-sitosterol (14.82%) and globulol (13.45%) are notable essential compounds in the headspace [59] (see Figure 2).
Recent research revealed the presence of significant phenolic and flavonoid compounds in T. inodorum, namely apigenin, apigenin-7-O-glucoside, luteolin, luteolin-7-O-glucoside, quinic acid, and 5-O-caffeoyl quinic acid [60] (Figure 3).
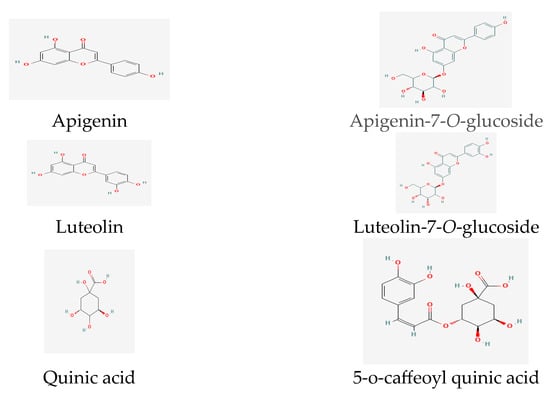
Figure 3. Phenolic and flavonoid compounds in Tripleurospermum spp.
Furthermore, recent reports indicated that the genus T. insularum Inceer & Hayırlıoglu-Ayaz [19] contains fatty acids and n-alkanes (38.43–59.22%), such as n-octacosane, n-hexacosane, and linoleic acid (Figure 4).
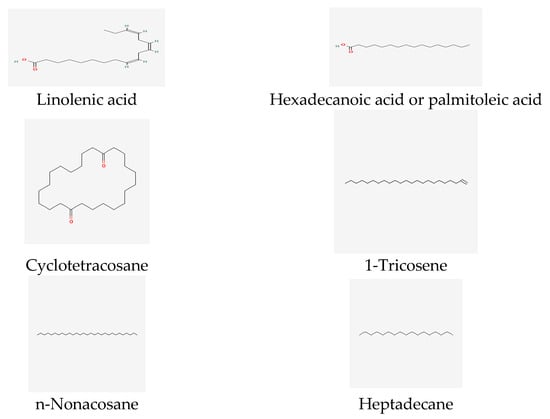
Figure 4. Fatty acids and hydrocarbon compounds of Tripleurospermum spp.
Plants belonging to the genus Tripleurospermum encompass a wide range of phytochemicals, including terpenoids, alkanes, steroids, organic acids, and aromatic compounds [61,62][61][62]. The essential oil of these plants is characterized by the presence of p-methoxy-β-cyclopropylstyrene, (E)-β-farnesene, β-sesquiphellandrene, and cis-calamenene as major components [63].
In the case of T. parthenium, extensive studies have evaluated its major compounds, which include many secondary metabolites, such as camphor, chrysanthenyl acetate, comphene, and bornyl acetate [64,65,66][64][65][66] (Figure 2).
In addition, the hot water extracts of T. parthenium and T. disciforme revealed the presence of melatonin in flowers [67]. Another study reported the detection of an acetylene derivative of dioxaspiran in the chloroform extract of T. disciforme [68].
The particularly volatile compounds of T. auriculatum from Saudi Arabia were characterized as fatty acids and their derivatives [69]. The main compounds of T. callosum chloroform extract have been identified as hexadecanoic and linoleic acids [57,63][57][63] (Figure 4), while the predominant compound in T. callosum is moretenol (11.71%) (Figure 2). Moreover, studies have demonstrated that the most common compounds in flower oil are linoleic acid (16.18%), n-hexadecanoic acid (17.88%) (Figure 4), and n-nonacosane (11.04%).
The major compound in the stem oil of T. callosum includes 1-tricosene (13.41%) (Figure 4), while the main compounds in the root oil of T. callosum are cyclotetracosane (5.88%) and n-hexadecanoic acid (6.18%) [62] (Figure 4).
Ref. [62] mentions that chloroform extracts of T. callosum flowers, stems, and roots include p-methoxy, β cyclopropylstyrene, and β-farnesene as the main compounds. Additionally, the essential oil of Tripleurospermum disciforme is predominantly composed of sesquiterpenes β-farnesene, p-methoxy-β-cyclopropyl styrene, β-sesquiphellandrene and cis-calamenene, ρ-methoxy-humulene oxide, benzene acetaldehyde (Figure 2), and heptadecane [57,59,63,70][57][59][63][70] (Figure 4).
A total of 21 compounds from T. disciforme essential oil were analyzed at different stages, including the flowering stages; and variations in the composition were observed. During the flowering stage, β-farnesene (22.46%) and β-sesquiphellandrene (17.85%) were found to be the major compounds, along with p-methoxy-β-cyclopropylstyrene (16.64%), heptadecane (10.60%) p-methoxy-humulene oxide (6.88%), and benzene acetaldehyde (9.30%) [63] (Figure 2).
The amounts of β-farnesene, β-sesquiphellandrene, p-methoxy-β-cyclopropylstyrene, heptadecane, and benzene acetaldehyde were reported to be highest during the flowering stage and decreased after flowering and seed maturation.
A total of 38 compounds from 89.4% of the total essential oil of T. parviflorum were identified. The essential oil of T. parviflorum was shown to contain significant compounds such as β-farnesene (18.4%), β-sesquiphellandrene (10.1%), carvacrol methyl ether (7.9%), and benzene acetaldehyde (7.2%) [71]. T. auriculatum was analyzed for flavonoids and sterols/triterpenes (90%), tannins (78%), volatile oils (60%), and alkaloids and coumarins (50%) [72]. T. parthenium was reported to contain a large proportion of oxidized monoterpenes [73]. In the essential oil of T. disciforme, the major compounds identified were nonterpenoids, including anisole, p-1-cyclohexen-1-yl- (55.95%), modephene (10.00%), camphor (43.43%), and cis-β-farnesene (11.94%). Additionally, the amount of caryophyllene (1.66%) was found to be higher than that in T. parthenium. [57,59,63,73][57][59][63][73].
It is worth noting that a study analyzing the chemical composition of T. disciforme [55] found a relatively small amount of β-esquiphellandrene (0.22%), which deviated from previous data. Another recent study [74] employed techniques such as NMR, electron impact mass spectroscopy, column chromatography, and thin layer- chromatography (see Figure 2) to identify three triterpenoids—taraxasterol, lupeol, and betulinic acid—in the dichloromethane extract of T. disciforme.
Regarding T. tenuifolium and T. parviflorum, palmitic acid (C 16:0) and linoleic acid (C 18:2) were identified as the main fatty acids (see Figure 4) [75]. Furthermore, saturated fatty acids (SFAs) were found to be present in higher amounts than monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) in both T. tenuifolium and T. Parviflorum.
References
- Zebarjadi, A.; Rostami Ahmadvandi, H.; Kahrizi, D.; Cheghamirza, K. Assessment of Genetic Diversity by Application of Inter Simple Sequence Repeat (ISSR) Primers on Iranian Harmal (Peganum harmala L.) Germplasm as an Important Medicinal Plant. J. Appl. Biotechnol. Rep. 2016, 3, 441–445.
- Chen, M.; He, X.; Sun, H.; Sun, Y.; Li, L.; Zhu, J.; Xia, G.; Guo, X.; Zang, H. Phytochemical analysis, UPLC-ESI-Orbitrap-MS analysis, biological activity, and toxicity of extracts from Tripleurospermum limosum (Maxim.) Pobed. Arab. J. Chem. 2022, 15, 103797.
- Inceer, H.; Hayirlioglu-Ayaz, S. Chromosome numbers in Tripleurospermum Sch. Bip.(Asteraceae) and closely related genera: Relationships between ploidy level and stomatal length. Plant Syst. Evol. 2010, 285, 149–157.
- Harling, G. Embryological Studies in the Compositae: 1–3; Odentryckeriet: Stockholm, Sweden, 1951.
- Rauschert, S. Nomenklatorische probleme in der gattung Matricaria L. Folia Geobot. Phytotaxon. 1974, 9, 249–260.
- Kay, Q. Chamomilla SF Gray and Matricaria L. Flora Eur. 1976, 4, 165–167.
- Garcia, S.; Inceer, H.; Garnatje, T.; Valles, J. Genome size variation in some representatives of the genus Tripleurospermum. Biol. Plant. 2005, 49, 381–387.
- Watanabe, W. Index to Chromosome Numbers in Asteraceae. 2021. Available online: http://www.lib.kobe-u.ac.jp/infolib/meta_pub/G0000003asteraceae_e (accessed on 27 July 2021).
- Fedorov, A. Chromosome numbers of flowering plants. Rept. Koenigstein. 1974. Available online: https://cir.nii.ac.jp/crid/1571698599030509440 (accessed on 12 March 2023).
- Hüser, W.; Hüser, W. Untersuchungen Über Die Anatomie und Wasserökologie Einiger Ostseestrandpflanzen; Springer: Berlin/Heidelberg, Germany, 1930.
- Bremer, K.; Anderberg, A.A. Asteraceae: Cladistics and Classification; TimberPress, Inc.: Portland, OR, USA, 1994; p. 752.
- Bremer, K. Generic monograph of the Asteraceae-Anthemideae. Bull. Nat. Hist. Mus. Lond. (Bot.) 1993, 23, 71–177.
- Mozaffarian, V. A dictionary of Iranian plant names. Tehran Farhang Moaser 1996, 396, 396–398.
- Mabberley, D.J. The Plant-Book: A Portable Dictionary of the Vascular Plants; Cambridge University Press: Cambridge, UK, 1997.
- Oberprieler, C.; Himmelreich, S.; Vogt, R. A new subtribal classification of the tribe Anthemideae (Compositae). Willdenowia 2007, 37, 89–114.
- Inceer, H.; Beyazoğlu, O. A new record for the Flora of Turkey: Tripleurospermum subnivale Pobed. (Asteraceae). Turk. J. Bot. 2004, 28, 599–601.
- Yildirimli, S. Some new taxa, records and taxonomic treatments from Turkey. Herb J. Syst. Bot. 2010, 17, 1–114.
- Inceer, H.; Hayirlioglu-Ayaz, S. Tripleurospermum ziganaense (Asteraceae, Anthemideae), a new species from north-east Anatolia, Turkey. Bot. J. Linn. Soc. 2008, 158, 696–700.
- Inceer, H.; Hayırlıoğlu-Ayaz, S. Tripleurospermum insularum (Asteraceae, Anthemideae), a new species from Turkey. In Annales Botanici Fennici; BioOne: Washington, DC, USA, 2014; pp. 49–53.
- Inceer, H.; Bal, M.; Ceter, T.; Pinar, N.M. Fruit structure of 12 Turkish endemic Tripleurospermum Sch. Bip.(Asteraceae) taxa and its taxonomic implications. Plant Syst. Evol. 2012, 298, 845–855.
- Özbek, M.U.; Onayli, H. A new variety of the Tripleurospermum (Asteraceae) from Turkey. Biyol. Çeşitlilik ve Koruma 2020, 13, 136–143.
- Inceer, H.; Ozcan, M. Taxonomic evaluations on the anatomical characters of leaf and achene in Turkish Tripleurospermum with its relative Matricaria (Asteraceae). Flora 2021, 275, 151759.
- Tekşen, M.; Erkul, S.K.; Duman, H.; Ateş, M.A.; SAĞIROĞLU, M. Tripleurospermum eskilensis (Asteraceae): A new halophytic species from CentralAnatolia, Turkey. Turk. J. Bot. 2022, 46, 160–175.
- POWO, R.B.G. Kew. Plants of the World Online. 2021. Royal Botanic Garden, Kew. Available online: http://plantsofheworldonline.org/ (accessed on 23 September 2021).
- Inceer, H.; Garnatje, T.; Hayırlıoğlu-Ayaz, S.; Pascual-Díaz, J.P.; Vallès, J.; Garcia, S. A genome size and phylogenetic survey of Mediterranean Tripleurospermum and Matricaria (Anthemideae, Asteraceae). PLoS ONE 2018, 13, e0203762.
- Pobedimova, E. Galium L. Flora USSR 2000, 23, 345–459.
- Podlech, D.; Rechinger, K. Tripleurospermum Sch. Bip. Flora Iranica 1986, 158, 73–80.
- Oberprieler, C. Phylogenetic relationships in Anthemis L. (Compositae, Anthemideae) based on nrDNA ITS sequence variation. Taxon 2001, 50, 745–762.
- Inceer, H.; Beyazoglu, O. Karyological studies in Tripleurospermum (Asteraceae, Anthemideae) from north-east Anatolia. Bot. J. Linn. Soc. 2004, 146, 427–438.
- Inceer, H.; Ozcan, M. Leaf anatomy as an additional taxonomy tool for 18 taxa of Matricaria L. and Tripleurospermum Sch. Bip.(Anthemideae-Asteraceae) in Turkey. Plant Syst. Evol. 2011, 296, 205–215.
- Chehregani, A.; Mahanfar, N. Achene micro-morphology of Anthemis (Asteraceae) and its allies in Iran with emphasis on systematics. Int. J. Agric. Biol. 2007, 9, 486–488.
- Özcan, M.; İnceer, H. Stem anatomical survey of the genera Matricaria and Tripleurospermum (Asteraceae) from Turkey with its taxonomical and ecological implications. Bot. Serbica 2022, 46, 143–151.
- Bingham, E. Stomatal chloroplasts in alfalfa at four ploidy levels 1. Crop Sci. 1968, 8, 509–510.
- Santen, E.; Casler, E. Evaluation of indirect ploidy indicators in Dactylis C. subspecies. Crop Sci. 1986, 26, 848–852.
- Mishra, M. Stomatal Characteristics at Different Ploidy Levels in Coffea L. Ann. Bot. 1997, 80, 689–692.
- Beck, S.L.; Dunlop, R.W.; Fossey, A. Stomatal length and frequency as a measure of ploidy level in black wattle, Acacia mearnsii (de Wild). Bot. J. Linn. Soc. 2003, 141, 177–181.
- Stebbins, G.L. Chromosomal evolution in higher plants. In Chromosomal Evolution in Higher Plants; Edward Arnold: London, UK, 1971.
- Seidler-Łożykowska, K. Determination of the ploidy level in chamomile (Chamomilla recutita (L.) Rausch.) strains rich in a-bisabolol. J. Appl. Genet. 2003, 44, 151–155.
- Kynclova, M. Comparative morphology of achenes of the tribe Anthemideae Cass. (Family Asteraceae) and its taxonomic significance. Preslia 1970, 42, 33–53.
- Källersjö, M. Fruit structure and generic delimitation of Athanasia (Asteraceae–Anthemideae) and related South African genera. Nord. J. Bot. 1985, 5, 527–542.
- Bruhl, J.J.; Quinn, C.J. Cypsela anatomy in the ‘Cotuleae’(Asteraceae–Anthemideae). Bot. J. Linn. Soc. 1990, 102, 37–59.
- Funk, V.A.; Susanna, A.; Steussy, T.F.; Robinson, H.E. Classification of compositae. In Systematics, Evolution, and Biogeography of Compositae; International Association for Plant Taxonomy (IAPT): Vienna, Austria, 2009; pp. 171–189.
- Presti, R.M.L.; Oppolzer, S.; Oberprieler, C. A molecular phylogeny and a revised classification of the Mediterranean genus Anthemis sl (Compositae, Anthemideae) based on three molecular markers and micromorphological characters. Taxon 2010, 59, 1441–1456.
- Kurkcuoglu, M.; Tosun, F.; Inceer, H.; Baser, K.H.C. Volatile Compounds of Tripleurospermum decipiens from Two Natural Populations in Turkey. Chem. Nat. Compd. 2019, 55, 565–567.
- Enayet Hossain, A. Tripleurospermum Schultz Bip. Flora Turk. East Aegean Isl. 1975, 5, 295–311.
- Inceer, H. Lectotypification of the name Chamaemelum heterolepis, the basionym of Tripleurospermum heterolepis and taxonomic notes on T. tempskyanum (Asteraceae). Bot. Serbica 2021, 45, 347–352.
- Anderberg, A.; Baldwin, B.; Bayer, R.; Breitwieser, J.; Jeffrey, C.; Dillon, M.; Eldenäs, P.; Funk, V.; Garcia-Jacas, N.; Hind, D. Compositae. In Flowering Plants · Eudicots; Springer: Berlin, Germany, 2007; pp. 61–588.
- Metcalfe, C.; Chalk, L. Anatomy of the Dicotyledons. Vol I. Systematic Anatomy of Leaf and Stem, with a Brief History of the Subject, 2nd ed.; Oxford University Press: Oxford, UK, 1979.
- Zarinkamar, F.; Saderi, Z.; Soleimanpour, S. Excluder Strategies in Response to Pb Toxicity in Matricaria chamomilla. Adv. Bioresearch 2013, 4, 39–49.
- Uysal, I. Tripleurospermum baytopianum E. Hossain ve Centaurea polyclada DC. endemik türlerinin morfolojisi, anatomisi ve ekolojisi üzerine araştırmalar. Anadolu Üniv. Fen-Edeb. Fakültesi Derg. 1991, 3, 37–51.
- Khayati, M.; Pakravan, M.; Sonboli, A. A new record of Tripleurospermum Sch. Bip.(Asteraceae) from Iran. Nova Biol. Reper. 2016, 3, 151–156.
- Bremer, K. Asteraceae cladistics and classification. Timber, Portland, Oreg. Cladistics 1994, 10, 295–304.
- Aiken, S. Flora of the Canadian Arctic Archipelago; NRC Research Press: Ottawa, ON, Canada, 2007.
- Raal, A.; Kaur, H.; Orav, A.; Arak, E.; Kailas, T.; Mueuerisepp, M. Content and composition of essential oils in some Asteraceae species. Proc. Est. Acad. Sci. 2011, 60, 55.
- Nazar Alipoor, A.; Sefidkon, F. Quantitative and qualitative study of the essential oil from aromatic and medicinal Tripleurospermum disciforme (CA Mey.) Schultz-Bip. J. Med. Plants 2003, 2, 33–40.
- Kurkcuoglu, M.; Tosun, F.; Inceer, H.; Baser, K.H.C. Volatile compounds of Tripleurospermum decipiens from different sites in Turkey. Planta Med. 2016, 82, P718.
- Javidnia, K.; Miri, R.; Soltani, M.; Khosravi, A. Essential oil composition of Tripleurospermum disciforme from Iran. Chem. Nat. Compd. 2008, 44, 800–801.
- Servi, H.; Yücel, Y.Y.; Polatoğlu, K. Composition and Acetylcholinesterase inhibition properties of Tripleurospermum inodorum (L.) Sch. Bip. Essential Oil from Istanbul. Aurum J. Health Sci. 2018, 1, 23–38.
- Ćavar Zeljković, S.; Ayaz, F.A.; Inceer, H.; Hayirlioglu-Ayaz, S.; Colak, N. Evaluation of chemical profile and antioxidant activity of Tripleurospermum insularum, a new species from Turkey. Nat. Prod. Res. 2015, 29, 293–296.
- Šibul, F.; Orčić, D.; Berežni, S.; Anačkov, G.; Mimica-Dukić, N. HPLC–MS/MS profiling of wild-growing scentless chamomile. Acta Chromatogr. 2020, 32, 86–94.
- Servi, H.; Sen, A.; Dogan, A. Chemical composition and biological activities of endemic Tripleurospermum conoclinium (Boiss. & Balansa) Hayek essential oils. Flavour Fragr. J. 2020, 35, 713–721.
- Yaşar, A.; Üçüncü, O.; Güleç, C.; İnceer, H.; Ayaz, S.; Yayl, N. GC-MS Analysis of Chloroform Extracts in Flowers, Stems, and Roots of Tripleurospermum callosum. Pharm. Biol. 2005, 43, 108–112.
- Chehregani, A.; Mohsenzadeh, F.; Mirazi, N.; Hajisadeghian, S.; Baghali, Z. Chemical composition and antibacterial activity of essential oils of Tripleurospermum disciforme in three developmental stages. Pharm. Biol. 2010, 48, 1280–1284.
- Rezaei, F.; Jamei, R.; Heidari, R. Evaluation of the phytochemical and antioxidant potential of aerial parts of Iranian Tanacetum parthenium. Pharm. Sci. 2017, 23, 136–142.
- Mojab, F.; Tabatabai, S.A.; Naghdi-Badi, H.; Nickavar, N.; Ghadyani, F. Essential oil of the root of Tanacetum parthenium (L.) Schulz. Bip.(Asteraceae) from Iran. Iran. J. Pharm. Res. 2010, 6, 291–293.
- Akpulat, H.A.; Tepe, B.; Sokmen, A.; Daferera, D.; Polissiou, M. Composition of the essential oils of Tanacetum argyrophyllum (C. Koch) Tvzel. var. argyrophyllum and Tanacetum parthenium (L.) Schultz Bip.(Asteraceae) from Turkey. Biochem. Syst. Ecol. 2005, 33, 511–516.
- Ansari, M.; Rafiee, K.; Yasa, N.; Vardasbi, S.; Naimi, S.; Nowrouzi, A. Measurement of melatonin in alcoholic and hot water extracts of Tanacetum parthenium, Tripleurospermum disciforme and Viola odorata. DARU J. Pharm. Sci. 2010, 18, 173.
- Souri, E.; Sarkhail, P.; Kaymanesh, P.; Amini, M.; Farsam, H. Antioxidant activity of extract and a new isolated dioxaspiran de-rivative of Tripleurospermum disciforme. Pharm. Biol. 2005, 43, 620–623.
- Alkhathalan, H.; AlHazimi, H. Chemical constituents of T-aurilactum, R-vesicarius, P-orientalis, P-somalensis and A-abyssinica grown in Saudi Arabia. J. Chem. Soc. Pak. 1996, 18, 309–312.
- Özturk, E.; Özer, H.; Çakir, A.; Mete, E.; Kandemir, A.; Polat, T. Chemical composition of the essential oil of Tripleurospermum corymbosum E. Hossain, an Endemic Species from Turkey. J. Essent. Oil Bear. Plants 2010, 13, 148–153.
- Kilic, O.; Bagci, E. Chemical composition of essential oil of Tripleurospermum parviflorum (Willd.) pobed (Asteraceae) from Turkey. Asian J. Chem. 2012, 24, 1319–1321.
- Tariq, M.; Mossa, J.; Al-Yahya, M.; Al-Meshal, I.; Al-Badr, A. Phytochemical and Biological Screening of Saudi Medicinal Plants Part-10* A Study on Saudi Plants of Family Compositae. Int. J. Crude Drug Res. 1987, 25, 17–25.
- Ghavam, M. Tripleurospermum disciforme (CA Mey.) Sch. Bip., Tanacetum parthenium (L.) Sch. Bip, and Achillea biebersteinii Afan.: Efficiency, chemical profile, and biological properties of essential oil. Chem. Biol. Technol. Agric. 2021, 8, 1–17.
- Ghoran, S.H.; Babaei, E.; Seresht, H.R.; Karimzadeh, Z. Cytotoxic constituents and molecular docking study of the active triterpenoids from Tripleurospermum disciforme (CA Mey.) Schultz-Bip. Jundishapur J. Nat. Pharm. Prod. 2020, 15, e65760.
- Ayaz, F.A.; Inceer, H.; Hayirlioglu-Ayaz, S.; Aksu-Kalmuk, N. Achene fatty acid composition in the tribe Anthemideae (Asteraceae). Rom. Biotechnol. Lett. 2016, 21, 11577.
More
