Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Peter Matúš and Version 2 by Rita Xu.
Pseudomonas
biofilms have been studied intensively for several decades and research outcomes have been successfully implemented in various medical and agricultural applications. Research on biofilm synthesis and composition has also overlapped with the objectives of environmental sciences, since biofilm components show exceptional physicochemical properties applicable to remediation techniques. Especially, exopolysaccharides (ExPs) have been at the center of scientific interest, indicating their potential in solving the environmental issues of heavy metal land and water contamination via sorptive interactions and flocculation.
- exopolysaccharides
- Pseudomonas
- biosorption
- bioremediation
1. Introduction
Bacterial exopolysaccharides (ExPs), a group of extracellular polymeric substances (EPSs), are the structural and functional components of microbial biofilms that display exceptional physicochemical properties. Thus, bacterial ExPs with unique attributes have found their way into biomedical science practice (e.g., tissue engineering) and have been successfully implemented into a myriad of industrial and medical applications [1][2][1,2].
Interest in ExP-producing bacteria has also expanded into the research areas of environmental sciences, including studies on eco-friendly municipal and wastewater treatment processes [3]. This is because many standard remediation techniques require the usage of reagents that may be hard to degrade, or various environmentally harmful by-products are produced during their utilization at contaminated sites. This includes chemical treatment, which usually detoxifies metals via redox transformation or neutralization by application of the reagents, such as potassium permanganate, hydrogen peroxide, hypochlorite, synthetic surfactants, or chlorine gas, to precipitate, immobilize, or preconcentrate the hazardous contaminants [4][5][6][4,5,6]. The chemical leaching of soils and sediments by applying strong inorganic and organic acids and persistent synthetic chelating agents (e.g., ethylenediaminetetraacetic acid or its derivatives) to solubilize contaminants has also been successfully tested for heavy metal removal [7]. Reactive solid inorganic and biological substances, as well as materials with active surfaces (e.g., zero-valent iron, ferric oxides and oxohydroxides, nanomaterials, zeolite, biological waste) have been studied as potential components of permeable treatment barriers to restrict the movement of the contaminant in the environment [8][9][10][11][8,9,10,11]. However, in some cases, unpredictable effects regarding the toxicity and mobility of generated species can be expected since these interactions are usually non-specific. The application of electrochemical and electrokinetic remediation methods, engineered to site-specific requirements, has been performed for heavy metal removal [12], showing promising results in combination with other remediation approaches, including novel biochemical methods [13]. More prominent green approaches include phytoremediation, phytoextraction, and biosorption. They are usually performed in conjunction with other methods, e.g., chemical leaching [14]. Still, the application of microbial ExPs is environmentally advantageous since these biogenic polymers are usually water soluble, susceptible to natural degradation, and less harmful than synthetic polymers [15].
Bacterial ExPs find their successful applications in heavy metal removal, oil recovery, and various in situ remediation techniques such as emulsifiers, sorbents, biofilters, surfactants, and bioflocculants [16][17][18][16,17,18]. The interest of environmental researchers in ExP-producing bacteria is also highlighted in several patent deposits focusing on the prosperous application of bacteria in the remediation of contaminated sites. Villela et al. [19] reported that there are 114 patents describing the degradation of oil compounds exclusively by Pseudomonas, thus highlighting the leading role of this bacterial genus in hydrocarbon-contaminated site remediation.
The utilization of Pseudomonas strains in remediation is not limited solely to the biodegradation of hydrocarbons; they have also been successfully applied for the decontamination of heavy metal-polluted waters, soils, and sediments [20]. This is primarily due to their ability to produce metal-chelating siderophores and surface-active extracellular polymeric substances [21]. Regarding the latter, Pseudomonas species are considered high-ExP-producing organisms [22], and since they are ubiquitous, being isolated from various types of environments, including industrial waste and activated sludge [23], they are considered potent in solving the issue of heavy metal contamination [24].
2. Biosynthesis of Extracellular Polysaccharides in Pseudomonas
Extensive progress has been made in elucidating the synthesis of bacterial extracellular homopolysaccharides and heteropolysaccharides in recent years (Figure 1). They are synthesized by bacteria either extracellularly (outside the cell membrane and the cell wall), within the cell wall, or intracellularly [25].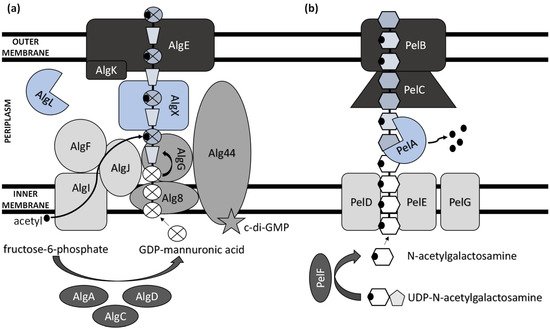
Figure 1. Schematic model of (a) alginate and (b) Pel polysaccharide biosynthetic machinery.
