Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Xiao Cheng and Version 2 by Conner Chen.
Photocatalytic overall water splitting in solar–chemical energy conversion can effectively mitigate environmental pollution and resource depletion. Stable ternary metal indium zinc sulfide (ZnIn2S4) is considered one of the ideal materials for photocatalytic overall water splitting due to its unique electronic and optical properties, as well as suitable conduction and valence band positions for suitable photocatalytic overall water splitting, and it has attracted widespread researcher interest.
- photocatalytic overall water splitting
- ZnIn2S4
- doping
- vacancy
1. Introduction
The world is currently suffering from environmental pollution and resource depletion, with energy issues looming large. According to relevant studies, the annual global consumption of energy is equivalent to the solar energy reaching the Earth’s surface every hour; therefore, solar energy as an abundant, non-polluting natural resource has replaced the traditional fuel fossil as a research hotspot [1]. However, solar energy has limitations such as intermittency and low density, so an effective storage method is needed to make efficient use of solar energy [2]. Since 1972, when it was reported that TiO2 semiconductors could produce hydrogen and oxygen when irradiated by ultraviolet light, photocatalysis, which uses solar energy to convert it into storable chemical energy, has attracted extensive research [3].
Hydrogen, as a clean, high-energy-density solar fuel, is the ideal energy carrier. Since most photocatalytic hydrogen production studies require the use of sacrificial agents to achieve this, photocatalytic overall water splitting is considered a low-cost, ideal method for converting solar energy into hydrogen energy [4][5][6][4,5,6]. The photocatalytic overall water splitting process is based on three fundamental photocatalytic processes: photocatalyst absorption of photons to generate electron–hole pairs, photogenerated charge transfer and separation, and surface redox reactions. A variety of semiconductor catalysts such as metal oxides, metal sulfides, and nitrides are currently used in the field of photocatalytic overall water splitting [7][8][9][10][11][12][7,8,9,10,11,12]. Among them, metal sulfides have the advantages of good charge transfer ability, suitable energy band structure for overall water splitting, and excellent light collection ability to become one of the potential catalysts in photocatalytic overall water splitting [13].
Metal sulfides are mainly classified into binary metal sulfides such as CdS, MoS2, and ZnS; ternary metal sulfides such as ZnIn2S4 and CuInS4; and polymetallic sulfides such as AgZnInS [14]. Most of these binary sulfides have some disadvantages that are more difficult to improve, such as ZnS-based photocatalysts having a poor photo-response, responding only to ultraviolet (UV) light, and CdS-based catalysts having severe photo-corrosion and poor stability, whereas ternary metal sulfides tend to be more stable [15][16][17][18][15,16,17,18]. Zinc indium sulfide (ZnIn2S4), a ternary metal sulfide belonging to the AB2X4 family, has unique electronic and optical properties. Compared with conventional photocatalysts, ZnIn2S4 has a narrower band gap, adjustable between about 2.06 and 2.85 eV, and has thermodynamically suitable conduction and valence band positions for photocatalytic overall water splitting as well as a strong visible-light response range [19][20][19,20]. In addition, ZnIn2S4 has many advantages such as strong photostability, relatively environmentally friendly chemical composition, ease of preparation, and wide distribution of raw materials [21]. Therefore, ZnIn2S4 is a more desirable material for photocatalytic overall water splitting.
Although ZnIn2S4 has many advantages, in practical applications, ZnIn2S4-based photocatalysts suffer from difficulties in achieving one-component photocatalytic overall water splitting or low photocatalytic overall water splitting efficiency, mainly due to the slow photo-generated charge separation and migration efficiency and weak solar energy utilization [22][23][24][22,23,24]. Therefore, appropriate modification strategies such as elemental doping, vacancy engineering, the construction of heterojunctions, and the loading of co-catalysts are required to improve the performance of ZnIn2S4-based photocatalyst materials.
Researchers have actively explored how to improve the performance of ZnIn2S4-based photocatalysts and have reported on a review of ZnIn2S4 photocatalysts from different perspectives. For example, Liu et al. reviewed the research progress of ZnIn2S4-based photocatalysts constructed with heterojunctions for photocatalytic hydrogen production [25]. Yadav et al. reviewed various modification strategies to improve the performance of ZnIn2S4-based photocatalysts and summarized their applications in water pollution treatment, CO2 reduction, etc. [26]. However, previous reports are mainly based on applications such as hydrogen production and pollutant treatment, and there is no systematic summary of the research progress on ZnIn2S4-based photocatalysts for achieving photocatalytic overall water splitting.
2. Mechanisms of Photocatalytic Overall Water Splitting
Photocatalytic overall water splitting consists of two half-reactions, the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). Theoretically, in order to achieve overall water splitting, the semiconductor band gap should be no less than 1.23 eV under standard conditions, the potential at the bottom of the conduction band should be less than 0 eV (H2/H+ = 0 eV vs. NHE, pH = 0), and that at the top of the valence band should be greater than 1.23 eV (H2O/O2 = 1.23 eV vs. NHE, pH = 0) [27]. The redox potential of water is all located within the band gap of the photocatalyst and photocatalytic overall water splitting is thermodynamically feasible. However, photocatalytic overall water splitting is an uphill reaction requiring additional energy to promote water splitting, which is a thermodynamically unfavorable process (G > 0); therefore, hydrogen and oxygen are prone to the reverse reaction and H2O reformation, which severely inhibits the photocatalytic water splitting activity [28]. Semiconductor-based photocatalysts for photocatalytic overall water splitting are based on three basic processes of photocatalysis: under solar irradiation with an energy greater than the band gap of the photocatalyst, photogenerated electrons are excited to leap to the conduction band and photogenerated holes remain in the valence band; photogenerated charges migrate separately to the semiconductor reaction site; and un-recombined photogenerated electrons and holes undergo redox reactions of water at the catalyst surface [29]. From the kinetic point of view, the recombination of photogenerated carriers is much faster than their redox reactions at the surface. The Coulomb force constraints between photogenerated charges and high interfacial potential barriers during charge transfer lead to rapid photogenerated carrier recombination and low utilization efficiency, which severely limit photocatalytic activity [30]. In addition, the range of solar energy utilization affects the photocatalytic activity. According to relevant research reports, the UV content of natural sunlight is less than 3%, the visible content is less than 40%, and the near-infrared occupies about 50% of the sunlight, while photocatalytic materials capture light basically in the UV and visible region, with a low efficiency of solar energy utilization [31][32][31,32]. The overall photocatalytic water splitting activity is limited by the low light collection capacity of the catalyst, the rate of photogenerated charge separation and migration, and the surface oxidation reaction [33]. Therefore, researchers have adopted corresponding modification strategies to prepare photocatalysts with high activity and high solar energy utilization efficiency. The stable ternary metal sulfide ZnIn2S4 is one of the ideal materials for photocatalytic overall water splitting due to its advantages. As shown in Scheme 1Scheme 2, the ZnIn2S4-based photocatalysts have thermodynamically suitable conduction and valence band positions for photocatalytic water splitting. However, single-component photocatalytic water splitting is difficult to achieve due to the overall low charge utilization and solar utilization as well as photo-corrosion phenomena. Therefore, modification strategies such as the doping of heteroatoms, formation of defects, construction of heterojunctions, and loading of co-catalysis were adopted to enhance the ZnIn2S4-based photocatalytic performance.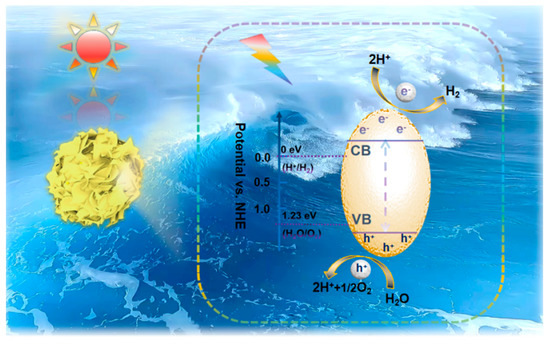
Scheme 12.
Schematic diagram of ZnIn
2
S
4
-based photocatalyst photocatalytic overall water splitting.
