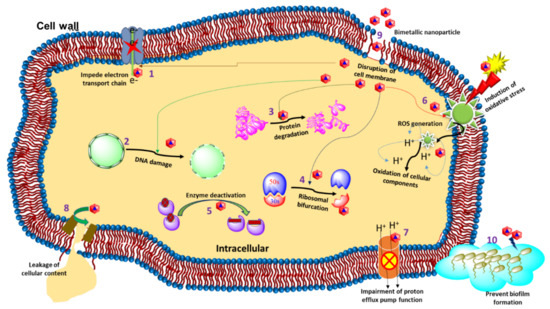
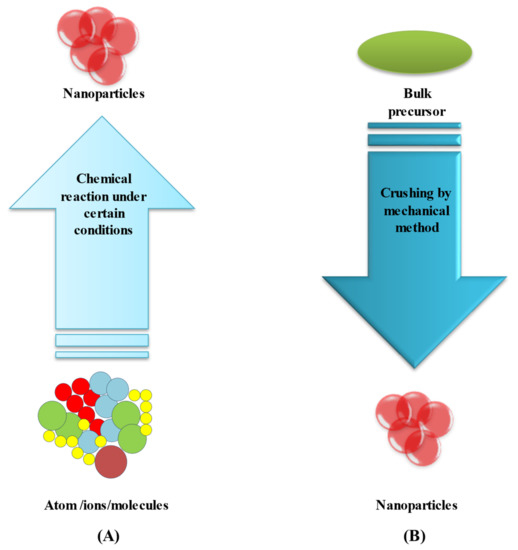
Bimetallic nanoparticles (NPs) with two separate metals have been found to have stronger antibacterial potential than their monometallic versions. This enhanced antibacterial efficiency of bimetallic nanoparticles is due to the synergistic effect of their participating monometallic counterparts. To distinguish between bacteria and mammals, the existence of diverse metal transport systems and metalloproteins is necessary for the use of bimetallic Au–Ag NPs, just like any other metal NPs. Due to their very low toxicity toward human cells, these bimetallic NPs, particularly gold–silver NPs, might prove to be an effective weapon in the arsenal to beat emerging drug-resistant bacteria. The cellular mechanism of bimetallic nanoparticles for antibacterial activity consists of cell membrane degradation, disturbance in homeostasis, oxidative stress, and the production of reactive oxygen species. The synthesis of bimetallic nanoparticles can be performed by a bottom-up and top-down strategy. The bottom-up technique generally includes sol-gel, chemical vapor deposition, green synthesis, and co-precipitation methods, whereas the top-down technique includes the laser ablation method.
- antibacterial
- bimetallic
- gold–silver
- multidrug resistance
- nanoparticles
- wound healing
1. Introduction
2. The Antibacterial Mode of Action of Au–Ag NPs
2.1. Cell Membrane Degradation
2.2. Disturbance in Homeostasis
2.3. Oxidative Stress and the Production of Reactive Oxygen Species (ROS)
3. Synthesis Routes of BIMETALLIC Au–Ag Nanoparticles
3.1. Bottom-Up Method
3.2. Top-Down Method
This method is used to transform bulk material into tiny nanoparticles. Top-down techniques are straightforward to employ. However, they are unsuccessful when producing irregularly shaped and very small particles. The main disadvantage of the top-down approach is the difficulty in acquiring a suitable particle size and shape [95,96][66][67]. Laser ablation is the most controllable top-down approach. Bulk material is treated with a laser beam (in this case, a bimetallic Au–Ag alloy). Under optimum conditions, well-dispersed bimetallic Au–Ag NPs can be synthesized, which can then be fractionated and surface-functionalized. A two-step synthesis, which involves laser irradiating a combination of silver and gold nanoparticles, is another alternative [97][68].4. Antibacterial Properties of Bimetallic Au–Ag NPs
Controlling the invasion of new bacterial infections, their increasing proliferative powers, and antibacterial resistance, all of which have major public health implications, necessitates the use of extremely potent antimicrobial agents. Due to their synergistic effects, broad spectrum of physiochemical properties, and various mechanisms of action, bimetallic nanoparticles synthesized by combining two distinct metals have recently emerged as having a promising antibacterial efficiency exceeding those of their monometallic counterparts. Consequently, Au–Ag bimetallic nanoparticles are of great importance in imaging, biomedical devices, and nanomedicine [107][69]. In a study, Ding et al., synthesized Au–Ag core–shell NPs via a chemical route and investigated their antimicrobial efficacy. In this study, they reported the aggregation of Au–Ag core–shell NPs onto the bacterial surface, which led to improved imaging because of the improved two-photon photoluminescence. These nanoparticles were found to have antibacterial action against S. aureus while being less harmful to human dermal fibroblasts [81][70]. On the other hand, Bankura et al., reported the use of dextran as a reducing agent for the synthesis of Au–Ag alloy NPs and investigated their antimicrobial efficacy. The antibacterial activity of a 0.1 mg/mL concentration of Ag-Au alloy NPs was found to be significant against bacteria (B. subtilis, B. cereus, E. coli, and P. aeruginosa) with zones of inhibition of 24, 21, 17, and 20 mm [108][71]. In another report, the author followed a photosynthetic route to synthesize Au–Ag alloy nanoparticles for the first time. The bioreduction material in the study was essential oil from Coleus aromaticus. Gram-negative E. coli and Gram-positive S. aureus were used to test the antibacterial efficacy of the photosynthesized Au–Ag alloy nanoparticles. An inhibitory zone of 28 mm for the alloy nanoparticles (synthesized with 150 µL essential oil) demonstrated their strong bactericidal activity against E. coli. An in vitro antioxidant assay of the herbal-deduced nanoparticles also exhibited intense free radical (superoxide, hydroxyl, and nitric oxide radicals) scavenging activity [109][72]. Similarly, Amina et al., prepared Au–Ag alloy nanoparticles by using a microwave-assisted technique that utilized an extract of Asparagus racemosus root. In addition, the green-synthesized bimetallic alloy nanoparticles were tested against five different bacterial strains (Bacillus subtilis (ATCC 6633), Escherichia coli (ATCC 25922), Klebsiella pneumonia (Urine), Pseudomonas aeruginosa (ATCC 27853), and Staphylococcus aureus (ATCC 25923). It was reported that P. aeruginosa and S. aureus strains were the most susceptible (highest zone of inhibition) towards Au–Ag alloy nanoparticles versus single metal nanoparticles synthesized with plant extract [110][73]. Additionally, Gopinath et al., synthesized green bimetallic (Au–Ag) nanoparticles by using Gloriosa superba aqueous leaf extract. It was demonstrated that the developed nanoparticles had higher antibacterial as well as antibiofilm activities against Gram-positive and Gram-negative bacteria. The authors found a significant zone of inhibition at 6.33 ± 0.33 mm and 5.33 ± 0.33 mm for B. subtilis and E. coli, respectively [111][74]. In another study, recently developed biosynthesized Au–Ag NPs without the incorporation of a surfactant or stabilizing agent. It was observed that when the pH of a solution with E. coli and Au ions was raised, Au nanomaterials were formed. Core–shell Au–Ag nanostructures were generated in an ordered manner after Ag ions combined with the Au core. The spectroscopic and microscopic analyses confirmed the structural composition of the biosynthetic bimetallic Au–Ag nanoparticles [121][75]. In a similar study, Liu et al., reported that their bimetallic NPs showed stronger application possibilities in the superfast colorimetric monitoring of H2O2, photothermal treatments, and antimicrobial therapy. Without using 3,3′,5,5′-tetramethylbenzidine or peroxidase, their bimetallic Au–Cu NPs were able to sense H2O2 quickly and calorimetrically [122][76]. Furthermore, Au–Ag NPs could improve antibacterial activity without increasing cytotoxicity, ensuring that silver could be used in clinical settings [107][69]. In recent studies, Kalwar et al., created Au–Ag-NP-decorated cellulose nanofibers. Cellulose acetate nanofibers were made by electrospinning, and alkaline hydrolysis was used to deacetylate them. The Au–Ag NPs were coated on the surface of cellulose nanofibers using a dipping process to create an excellent wound dressing material. Furthermore, their antibacterial activity against E. coli and S. aureus was tested, and the Au–Ag NPs/cellulose was found to be a good antimicrobial material [123][77]. Villalobos-Noriega et al., synthesized bimetallic core–shell Au–Ag NPs by a green approach. Root extract of Rumex hymenosepalus containing catechins and stilbenes acted as a reducing agent in the NPs synthesis. The growth kinetics of microorganisms was analyzed by the Gompertz model. The findings suggested that silver NPs and bimetallic Au–Ag NPs had a dose-dependent effect on the lag phase and growth rate of E. coli and Candida albicans, with the Au–Ag NPs having a better response [120,124][78][79].5. Bimetallic Nanoparticles Targeting Multidrug-Resistant Bacteria
Multidrug-resistant (MDR) bacteria are widely recognized as one of the most serious current public health issues, killing an estimated 700,000 people each year throughout the world [125][80]. Furthermore, treating MDR bacteria with ineffective antibiotics promotes the expansion of bacterial tolerance. For example, almost more than 50% of S. aureus strains obtained from several US hospitals are methicillin-resistant, with some strains also being resistant to vancomycin and carbapenems [126][81]. MDR microorganisms are frequently linked to nosocomial infection. Some MDR bacteria, on the other hand, have become common sources of community-acquired illnesses. This is a significant breakthrough since community-wide MDR bacteria dissemination leads to a significant rise in the population at risk and causes an increase in the number of MDR-bacteria-related diseases. When the incidence of resistance patterns in bacteria causing community-acquired infections exceeds a certain threshold, broad-spectrum antibacterial and/or combination antibacterial therapy is indicated for the empiric treatment of community-acquired disorders. Efforts to combat drug-resistant diseases are being hampered by the sluggish discovery of new antibiotics. It is anticipated that there will be no effective antibiotics available by 2050 if no new antibiotics are discovered [127][82]. Due to the lack of effective antibiotics against MDR bacteria, developing nanoparticles has been used as a substitute. It has also been observed that bimetallic NPs are efficient against bacteria, including MDR bacteria [128][83]. Several studies have shown bimetallic NPs to be effective against MDR bacteria. When monometallic counterparts were joined to form bimetallic NPs, the antibacterial activity was increased [129][84]. In a study, Wang et al., reported that Au NPs and mercaptophenylboronic acid (MBA) are incapable of acting as antibiotics separately. However, when MBA was coupled with Au NPs, the Au–MBA NPs showed significant antibacterial activity against Gram-positive MDR clinical isolates (e.g., MDR Staphyloccocus aureus and MDR Staphyloccocus epidermidis) [130][85]. In a similar study, Zhao and his collaborators synthesized bimetallic NPs by combining two salt solutions in an aqueous phase and reducing them with sodium borohydride. Monometallic NPs were also synthesized by the same method as the corresponding salt for comparison. The antibacterial NPs’ MIC (minimal inhibitory concentration) against E. coli and S. aureus were determined. Out of the nine different synthesized bimetallic NPs screened, two types of bimetallic NPs, namely the AuRh and AuRu NPs, showed MICs of 7 and 20 µg/mL, respectively, against E. coli and S. aureus. All of these bimetallic NPs were ineffective against S. aureus, with the MIC for S. aureus exceeding 128 µg/mL [131][86]. Kumar and his colleagues developed carbohydrate-coated bimetallic Au–Ag NPs, which were more effective against MDR strains than their monometallic counterparts (i.e., Ag NPs and Au NPs). The Au–Ag NPs were significantly more capable against Gram-negative MDR E. coli and Enterobacter cloacae than standard antibiotics. An in vivo study also exhibited that bimetallic Au–Ag NPs were almost 11,000 times more effective than Gentamicin at killing MDR MRSA infecting mice skin wounds. The Au–Ag NPs could heal and regenerate the infected wounds faster and without scarring. The in vivo results showed that Au–Ag NPs are an effective antibacterial agent against MDR strains with no adverse side effects [82][87]. Other forms of Au–Ag bimetallic NPs have been investigated and their antibacterial activity studied, although mostly as coating agents rather than as a delivery method [132][88].6. Gold, Silver, and Gold–Silver Nanomaterials for Wound Healing
Wound healing is a complex biological process involving a series of cellular and molecular interactions targeted at repairing the injured tissue and restoring its protective function. The wound healing process occurs simultaneously in four different steps: hemostasis, inflammation, proliferation, and remodeling, all of which occur simultaneously. Various medications are now available on the market that can aid with wound healing. For wound healing, drugs that target blood coagulation, inflammatory reactions, platelet function, and cell proliferation are often employed. Glucocorticoids, nonsteroidal anti-inflammatory drugs, and chemotherapeutic agents are examples of these medications [82,133,134][87][89][90]. Bimetallic Au–Ag NPs are attractive candidates for wound dressing integration due to their high antibacterial potential and reduced toxicity profile compared to monometallic silver and gold NPs. According to Mârza et al., the antibacterial characteristics of silver can impact the healing process of skin regeneration, and in the meantime, the antibacterial properties of silver can assist an open wound by avoiding bacterial infection [135][91].References
- Lopatkin, A.J.; Bening, S.C.; Manson, A.L.; Stokes, J.M.; Kohanski, M.A.; Badran, A.H.; Earl, A.M.; Cheney, N.J.; Yang, J.H.; Collins, J.J. Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science 2021, 371, eaba0862.
- Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655.
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284.
- Mott, D.M.; Anh, D.T.N.; Singh, P.; Shankar, C.; Maenosono, S. Electronic transfer as a route to increase the chemical stability in gold and silver core–shell nanoparticles. Adv. Colloid Interface Sci. 2012, 185, 14–33.
- Yang, J.; Zhao, Y.; Cao, J.; Gong, C.; Zuo, J.; Zhang, N.; Zhao, Y. Hyaluronic acid and antimicrobial peptide-modified gold/silver hybrid nanocages to combat bacterial multidrug resistance. Int. J. Pharm. 2020, 586, 119505.
- Mahmudin, L.; Suharyadi, E.; Utomo, A.B.S.; Abraha, K. Optical properties of silver nanoparticles for surface plasmon resonance (SPR)-based biosensor applications. J. Mod. Phys. 2015, 6, 1071.
- Rossi, A.; Zannotti, M.; Cuccioloni, M.; Minicucci, M.; Petetta, L.; Angeletti, M.; Giovannetti, R. Silver Nanoparticle-Based Sensor for the Selective Detection of Nickel Ions. Nanomaterials 2021, 11, 1733.
- Feng, L.; Gao, G.; Huang, P.; Wang, K.; Wang, X.; Luo, T.; Zhang, C. Optical properties and catalytic activity of bimetallic gold-silver nanoparticles. Nano Biomed. Eng. 2010, 2, 258–267.
- Al-Zaban, M.I.; AlHarbi, M.A.; Mahmoud, M.A.; Bahatheq, A.M. Production of biodiesel from oleaginous fungal lipid using highly catalytic bimetallic gold-silver core-shell nanoparticle. J. Appl. Microbiol. 2021, 132, 381–389.
- Reñones, P.; Collado, L.; Iglesias-Juez, A.; Oropeza, F.E.; Fresno, F.; de la Peña, O.S. Silver-gold bimetal-loaded TiO2 Photocatalysts for CO2 Reduction; U.S. Department of Agriculture: Washington, DC, USA, 2020.
- Aazam, E.S.; Zaheer, Z. Silver bimetallic nanoparticles: Fabrication and removal of toxic chromium (VI). J. Mater. Sci. Mater. Electron. 2021, 32, 11043–11058.
- Jia, X.; Yao, Y.; Yu, G.; Qu, L.; Li, T.; Li, Z.; Xu, C. Synthesis of gold-silver nanoalloys under microwave-assisted irradiation by deposition of silver on gold nanoclusters/triple helix glucan and antifungal activity. Carbohydr. Polym. 2020, 238, 116169.
- Fereja, S.L.; Li, P.; Guo, J.; Fang, Z.; Zhang, Z.; Zhuang, Z.; Zhang, X.; Liu, K.; Chen, W. Silver-enhanced fluorescence of bimetallic Au/Ag nanoclusters as ultrasensitive sensing probe for the detection of folic acid. Talanta 2021, 233, 122469.
- Kazancioglu, E.O.; Aydin, M.; Arsu, N. Photochemical synthesis of bimetallic gold/silver nanoparticles in polymer matrix with tunable absorption properties: Superior photocatalytic activity for degradation of methylene blue. Mater. Chem. Phys. 2021, 269, 124734.
- GÜRSOY, N. Fungus-mediated synthesis of silver nanoparticles (agnp) and inhibitory effect on Aspergillus spp. in combination with antifungal agent. Cumhur. Sci. Journa. 2020, 41, 311–318.
- Dat, N.M.; Khang, P.T.; Anh, T.N.M.; Quan, T.H.; Thinh, D.B.; Thien, D.T.; Nam, H.M.; Phong, M.T.; Hieu, N.H. Synthesis, characterization, and antibacterial activity investigation of silver nanoparticle-decorated graphene oxide. Mater. Lett. 2021, 285, 128993.
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. A sol–gel based silver nanoparticle/polytetrafluorethylene (AgNP/PTFE) coating with enhanced antibacterial and anti-corrosive properties. Appl. Surf. Sci. 2021, 535, 147675.
- Gao, M.; Sun, L.; Wang, Z.; Zhao, Y. Controlled synthesis of Ag nanoparticles with different morphologies and their antibacterial properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 397–404.
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. A facile method to prepare size-tunable silver nanoparticles and its antibacterial mechanism. Adv. Powder Technol. 2018, 29, 407–415.
- Asharani, P.; Wu, Y.L.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19, 255102.
- Shehata, A.M.; Salem, F.M.; El-Saied, E.M.; Abd El-Rahman, S.S.; Mahmoud, M.Y.; Noshy, P.A. Evaluation of the ameliorative effect of zinc nanoparticles against silver nanoparticle–induced toxicity in liver and kidney of rats. Biol. Trace Elem. Research 2021, 200, 1201–1211.
- Soares, T.; Ribeiro, D.; Proença, C.; Chisté, R.C.; Fernandes, E.; Freitas, M. Size-dependent cytotoxicity of silver nanoparticles in human neutrophils assessed by multiple analytical approaches. Life Sci. 2016, 145, 247–254.
- Jaswal, T.; Gupta, J. A review on the toxicity of silver nanoparticles on human health. Mater. Today Proc. 2021, in press.
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449.
- Ferdous, Z.; Nemmar, A. Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020, 21, 2375.
- Li, T.; Albee, B.; Alemayehu, M.; Diaz, R.; Ingham, L.; Kamal, S.; Rodriguez, M.; Bishnoi, W.S. Comparative toxicity study of Ag, Au, and Ag-Au bimetallic nanoparticles on Daphnia magna. Anal. Bioanal. Chem. 2010, 398, 689–700.
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul Toxicol. Pharmacol. 2014, 68, 1–7.
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci. Rep. 2020, 10, 9362.
- Ahmed, D.S.; Mohammed, M.K. Studying the bactericidal ability and biocompatibility of gold and gold oxide nanoparticles decorating on multi-wall carbon nanotubes. Chem. Pap. 2020, 74, 4033–4046.
- Ni, Z.; Gu, X.; He, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Synthesis of silver nanoparticle-decorated hydroxyapatite () poriferous nanocomposites and the study of their antibacterial activities. RSC Adv. 2018, 8, 41722–41730.
- Zhang, Z.; Wu, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Fabrication of silver nanoparticles embedded into polyvinyl alcohol (ag/pva) composite nanofibrous films through electrospinning for antibacterial and surface-enhanced raman scattering (sers) activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 462–469.
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical intermediate-modified gold nanoparticles: Against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 2017, 11, 5737–5745.
- Jana, S.K.; Gucchait, A.; Paul, S.; Saha, T.; Acharya, S.; Hoque, K.M.; Misra, A.K.; Chatterjee, B.K.; Chatterjee, T.; Chakrabarti, P. Virstatin-conjugated gold nanoparticle with enhanced antimicrobial activity against the vibrio cholerae el tor biotype. ACS Appl. Bio Mater. 2021, 4, 3089–3100.
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic nanoparticles for antimicrobial applications. Front. Chem. 2020, 8, 412.
- Kumar, S.; Majhi, R.K.; Singh, A.; Mishra, M.; Tiwari, A.; Chawla, S.; Guha, P.; Satpati, B.; Mohapatra, H.; Goswami, L.; et al. Carbohydrate-coated gold–silver nanoparticles for efficient elimination of multidrug resistant bacteria and in vivo wound healing. ACS Appl. Mater. Interfaces 2019, 11, 42998–43017.
- Bahrami, K.; Nazari, P.; Nabavi, M.; Golkar, M.; Almasirad, A.; Shahverdi, A.R. Hydroxyl capped silver-gold alloy nanoparticles: Characterization and their combination effect with different antibiotics against Staphylococcus aureus. Nanomed. J. 2014, 1, 155–161.
- Medina-Cruz, D.; Saleh, B.; Vernet-Crua, A.; Nieto-Argüello, A.; Lomelí-Marroquín, D.; Vélez-Escamilla, L.Y.; Cholula-Díaz, J.L.; García-Martín, J.M.; Webster, T. Bimetallic nanoparticles for biomedical applications: A review. In Racing for the Surface; Springer: Berlin/Heidelberg, Germany, 2020; pp. 397–434.
- Guo, B.; Alivio, T.E.; Fleer, N.A.; Feng, M.; Li, Y.; Banerjee, S.; Sharma, V.K. Elucidating the role of dissolved organic matter and sunlight in mediating the formation of Ag–Au bimetallic alloy nanoparticles in the aquatic environment. Environ. Sci. Technol. 2021, 55, 1710–1720.
- Simon, J.; Nampoori, V.; Kailasnath, M. Concentration dependent thermo-optical properties and nonlinear optical switching behavior of bimetallic Au-Ag nanoparticles synthesized by femtosecond laser ablation. Opt. Laser Technol. 2021, 140, 107022.
- Wang, M.; Zhou, X.; Wang, X.; Wang, M.; Su, X. One-step fabrication of wavelength-tunable luminescence of gold-silver bimetallic nanoclusters: Robust performance for α-glucosidase assay. Sens. Actuators B Chem. 2021, 345, 130407.
- Navya, P.; Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Maruyama, M.; Srinivas, S.P.; Jain, D.; Amin, M.H.; Bhargava, S.K.; Daima, H.K. Single step formation of biocompatible bimetallic alloy nanoparticles of gold and silver using isonicotinylhydrazide. Mater. Sci. Eng. C 2019, 96, 286–294.
- Mohsin, M.; Jawad, M.; Yameen, M.A.; Waseem, A.; Shah, S.H.; Shaikh, A.J.J.P. An insight into the coating behavior of bimetallic silver and gold core-shell nanoparticles. Plasmonics 2020, 15, 1599–1612.
- Sierra, M.A.; Casarrubios, L.; de la Torre, M.C. Bio-organometallic derivatives of antibacterial drugs. Chemistry 2019, 25, 7232–7242.
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327, 349–359.
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent developments in the medicinal applications of silver-nhc complexes and imidazolium salts. Molecules 2017, 22, 1263.
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441.
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733.
- Zhang, D.; Ma, X.L.; Gu, Y.; Huang, H.; Zhang, G.W. Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Front. Chem. 2020, 8, 799.
- Jena, P.; Bhattacharya, M.; Bhattacharjee, G.; Satpati, B.; Mukherjee, P.; Senapati, D.; Srinivasan, R. Bimetallic gold-silver nanoparticles mediate bacterial killing by disrupting the actin cytoskeleton MreB. Nanoscale 2020, 12, 3731–3749.
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361.
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815.
- Kim, Y.H.; Lee, D.K.; Cha, H.G.; Kim, C.W.; Kang, Y.C.; Kang, Y.S. Preparation and characterization of the antibacterial Cu nanoparticle formed on the surface of SiO2 nanoparticles. J. Phys. Chem. B 2006, 110, 24923–24928.
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152.
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotech. 2017, 15, 65.
- Wang, Y.; Malkmes, M.J.; Jiang, C.; Wang, P.; Zhu, L.; Zhang, H.; Zhang, Y.; Huang, H.; Jiang, L. Antibacterial mechanism and transcriptome analysis of ultra-small gold nanoclusters as an alternative of harmful antibiotics against Gram-negative bacteria. J. Hazard. Mater. 2021, 416, 126236.
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial gold nanoclusters. ACS Nano 2017, 11, 6904–6910.
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78.
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6.
- Keren, I.; Wu, Y.; Inocencio, J.; Mulcahy, L.R.; Lewis, K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 2013, 339, 1213–1216.
- Boonstra, J.; Post, J.A. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 2004, 337, 1–13.
- Długosz, O.; Sochocka, M.; Ochnik, M.; Banach, M. Metal and bimetallic nanoparticles: Flow synthesis, bioactivity and toxicity. J. Colloid. Interface Sci. 2021, 586, 807–818.
- Nasrabadi, H.T.; Abbasi, E.; Davaran, S.; Kouhi, M.; Akbarzadeh, A. Bimetallic nanoparticles: Preparation, properties, and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 376–380.
- Devi, N.; Sahoo, S.; Kumar, R.; Singh, R.K. A review of the microwave-assisted synthesis of carbon nanomaterials, metal oxides/hydroxides and their composites for energy storage applications. Nanoscale 2021, 13, 11679–11711.
- Fujimoto, K.T.; McMurtrey, M.D. Development of Bismuth and Platinum Bi-Metallic Nanoparticles to Enhance Melt Wire Temperature Resolution, Idaho National Lab. (INL), Idaho Falls, ID (United States). 2021. Available online: https://www.osti.gov/biblio/1813574 (accessed on 16 September 2022).
- Kawai, S.; Mardis, M.; Machmudah, S.; Kanda, H.; Zhao, Y.; Goto, M. Bimetallic nanoparticle generation from Au− TiO2 film by pulsed laser ablation in an aqueous medium. Alex. Eng. J. 2021, 60, 2225–2234.
- Sun, L.; Guan, J.; Xu, Q.; Yang, X.; Wang, J.; Hu, X. Synthesis and Applications of Molecularly Imprinted Polymers Modified TiO2 Nanomaterials: A Review. Polymers 2018, 10, 1248.
- Fu, X.; Cai, J.; Zhang, X.; Li, W.D.; Ge, H.; Hu, Y. Top-down fabrication of shape-controlled, monodisperse nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2018, 132, 169–187.
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser synthesis and processing of colloids: Fundamentals and applications. Chem. Rev. 2017, 117, 3990–4103.
- Mehata, A.K.; Suseela, M.N.L.; Gokul, P.; Malik, A.K.; Viswanadh, M.K.; Singh, C.; Selvin, J.; Muthu, M.S. Fast and highly efficient liquid chromatographic methods for qualification and quantification of antibiotic residues from environmental waste. Microchem. J. 2022, 179, 107573.
- Ding, X.; Yuan, P.; Gao, N.; Zhu, H.; Yang, Y.Y.; Xu, Q.H. Au-Ag core-shell nanoparticles for simultaneous bacterial imaging and synergistic antibacterial activity. Nanomedicine 2017, 13, 297–305.
- Bankura, K.; Rana, D.; Mollick, M.M.; Pattanayak, S.; Bhowmick, B.; Saha, N.R. Dextrin-mediated synthesis of Ag NPs for colorimetric assays of Cu(2+) ion and Au NPs for catalytic activity. Int. J. Biol. Macromol. 2015, 80, 309–316.
- Abbasi, B.H.; Zaka, M.; Hashmi, S.S.; Khan, Z. Biogenic synthesis of Au, Ag and Au–Ag alloy nanoparticles using Cannabis sativa leaf extract. IET Nanobiotechnol. 2018, 12, 277–284.
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Al-Hamoud, G.A. Antibacterial and immunomodulatory potentials of biosynthesized Ag, Au, Ag-Au bimetallic alloy nanoparticles using the asparagus racemosus root extract. Nanomaterials 2020, 10, 2453.
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Mohan, S.; Venkatesh, K.S.; Esakkirajan, M.; Kaleeswarran, P.; Alharbi, N.S.; Kadaikunnan, S.; Govindarajan, M.; et al. Green synthesis of silver, gold and silver/gold bimetallic nanoparticles using the Gloriosa superba leaf extract and their antibacterial and antibiofilm activities. Microb. Pathog. 2016, 101, 1–11.
- Singh, R.; Nawale, L.; Arkile, M.; Wadhwani, S.; Shedbalkar, U.; Chopade, S.; Sarkar, D.; Chopade, B.A. Phytogenic silver, gold, and bimetallic nanoparticles as novel antitubercular agents. Int. J. Nanomed. 2016, 11, 1889.
- Liu, C.; Im, S.H.; Yu, T. Synthesis of au–cu alloy nanoparticles as peroxidase mimetics for H2O2 and glucose colorimetric detection. Catalysts 2021, 11, 343.
- Kalwar, K.; Xi, J.; Ren, C.; Shen, M. Coating of on electrospun cellulose nanofibers for wound healing and antibacterial activity. Korean J. Chem. Eng. 2022, 39, 2165–2171.
- Villalobos-Noriega, J.M.A.; Rodríguez-León, E.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Plascencia-Jatomea, M.; Martínez-Higuera, A.; Martínez-Higuera, A.; Acuña-Campa, H.; García-Galaz, A.; Mora-Monroy,, B.; et al. nanoparticles synthesized with Rumex hymenosepalus as antimicrobial agent. Nanoscale Res. Lett. 2021, 16, 118.
- Samal, A.K.; Polavarapu, L.; Rodal-Cedeira, S.; Liz-Marzan, L.M.; Perez-Juste, J.; Pastoriza-Santos, I. Size Tunable Ag core-shell nanoparticles: Synthesis and surface-enhanced raman scattering properties. Langmuir 2013, 29, 15076–15082.
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15.
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283.
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-resistant bacteria and alternative methods to control them: An overview. Microb. Drug Resist. 2019, 25, 890–908.
- Zhang, M.; Wang, P.; Sun, H.; Wang, Z. Superhydrophobic surface with hierarchical architecture and bimetallic composition for enhanced antibacterial activity. ACS Appl. Mater. Interfaces 2014, 6, 22108–22115.
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Exp. Opin. Drug Deliv. 2010, 7, 753–763.
- Wang, L.; Yang, J.; Yang, X.; Hou, Q.; Liu, S.; Zheng, W.; Long, Y.; Jiang, X. Mercaptophenylboronic acid-activated gold nanoparticles as nanoantibiotics against multidrug-resistant bacteria. ACS Appl. Mater. Interfaces 2020, 12, 51148–51159.
- Zhao, X.; Jia, Y.; Dong, R.; Deng, J.; Tang, H.; Hu, F.; Liu, S.; Jiang, X. Bimetallic nanoparticles against multi-drug resistant bacteria. Chem Commun. 2020, 56, 10918–10921.
- Narendra; Mehata, A.K.; Viswanadh, M.K.; Sonkar, R.; Pawde, D.M.; Priya, V.; Singh, M.; Koch, B.; Muthu, M. Formulation and in vitro evaluation of upconversion nanoparticle-loaded liposomes for brain cancer. Ther. Deliv. 2020, 11, 557–571.
- Argueta-Figueroa, L.; Morales-Luckie, R.A.; Scougall-Vilchis, R.J.; Olea-Mejía, O.F. Synthesis, characterization and antibacterial activity of copper, nickel and bimetallic Cu–Ni nanoparticles for potential use in dental materials. Prog. Nat. Sci. Mater. Int. 2014, 24, 321–328.
- He, J.; Qiao, Y.; Zhang, H.; Zhao, J.; Li, W.; Xie, T.; Zhong, D.; Wei, Q.; Hua, S.; Yu, Y.; et al. Gold-silver nanoshells promote wound healing from drug-resistant bacteria infection and enable monitoring via surface-enhanced Raman scattering imaging. Biomaterials 2020, 234, 119763.
- Wang, X.; Guo, J.; Zhang, Q.; Zhu, S.; Liu, L.; Jiang, X.; Wei, D.H.; Liu, R.S.; Li, L. Gelatin sponge functionalized with gold/silver clusters for antibacterial application. Nanotechnology 2020, 31, 134004.
- Mârza, S.M.; Magyari, K.; Bogdan, S.; Moldovan, M.; Peștean, C.; Nagy, A.; Gal, A.F.; Tăbăran, F.; Purdoiu, R.C.; Licărete, E.; et al. The impact of composites with silicate-based glasses and gold nanoparticles on skin wound regeneration. Molecules 2021, 26, 620.
