Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Karolina Henryka Czarnecka-Chrebelska and Version 2 by Sirius Huang.
Chronic obstructive pulmonary disease (COPD) is one of the most prevalent chronic adult diseases, with significant worldwide morbidity and mortality. Although long-term tobacco smoking is a critical risk factor for this global health problem, its molecular mechanisms remain unclear. Several phenomena are thought to be involved in the evolution of emphysema, including airway inflammation, proteinase/anti-proteinase imbalance, oxidative stress, and genetic/epigenetic modifications. Furthermore, COPD is one main risk for lung cancer (LC), the deadliest form of human tumor; formation and chronic inflammation accompanying COPD can be a potential driver of malignancy maturation.
- chronic obstructive pulmonary disease
- lung cancer
- biomarkers
- smokers
- emphysema
1. Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by lung airflow limitation and tissue destruction; it is the third leading cause of death worldwide [1]. COPD can result from chronic bronchitis with characteristic airway inflammation and scarring [2]. Tobacco smoking is the most common COPD risk factor. However, many other inhaled irritants are still involved (burning biomass fuels, smoke, air pollutants, and chemicals), leading to heterogeneous COPD phenotypes [3][4][3,4].
2. COPD Causes
Chronic obstructive pulmonary disease (COPD) involves the chronic inflammatory condition of the lung, particularly the conducting airways and parenchyma [5][8]. Imbalances accompany the process of progressive inflammation in proteinase/anti-proteinase activity or oxidant–antioxidant balance, triggering emphysema formation (the abnormal enlargement of the air spaces located peripherally from the terminal bronchioles and destruction of the walls of these structures) [1][2][3][1,2,3]. Emphysema may lead to further changes in lung tissue, i.e., deterioration of elasticity, poor expiratory flow, gas trapping, and impairment of the gas exchange [2]. COPD affects millions worldwide, making it a significant health burden connected with high healthcare costs [6][9]. COPD is attributed to increasing morbidity and mortality in low- and middle-income countries, especially in acute exacerbation patients [7][10]. The impact of the COPD issue is that many people are underdiagnosed, and only 50% of patients are adequately treated with medications [8][11]. Tobacco smoking is the most common risk factor for developing COPD, with patients more likely to develop the disease if they smoked one pack per day for 20 years or more [3]. However, the absolute risk of COPD developing in continuous smokers is around 25%, suggesting that there may be other predisposing factors, such as genetic, epigenetic, or host-dependent factors (Figure 1).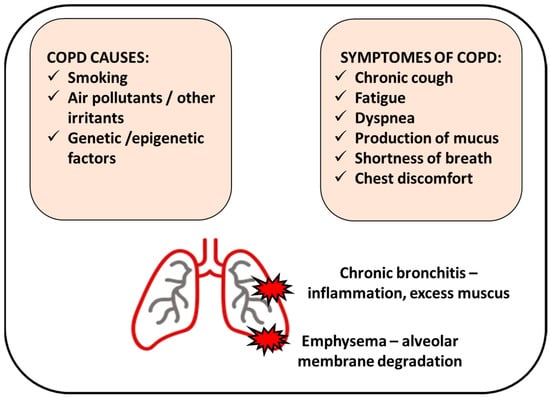
Figure 1. Chronic obstructive pulmonary disease (COPD) manifests by inflamed airways and damaged lung tissue. Smoking cigarettes is the most common cause of COPD; however, other factors can be involved. COPD includes chronic bronchitis (inflammation of the bronchial tubes that causes a persistent cough) and emphysema (damage of the air sacs).
3. Genetics of COPD
3.1. Gene Polymorphisms
Genome-wide association studies have been conducted to find the genes responsible for the onset or progression of COPD [22][25] (Table 1). One detected gene directly responsible for COPD appearance is alpha-1-antitrypsin (AAT) [23][26]. AAT deficiency, due to alterations in DNA sequence, is associated with disease development in 1–2% of the affected population. Occurrence of specific alleles, ATT*Z allele homozygosity (Pi Z) or heterozygosity of the Z allele with a null allele, is related with AAT deficiency. In Pi Z homozygotes, the AAT protein polymerize reduces the amount of protein circulating in the body and causes a decreased serum level of AAT [24][27]. The accumulation of AAT in the hepatocytes leads to liver disorders, such as cirrhosis, hepatitis, and cancer [25][28]. Since AAT acts as a plasma protease inhibitor of the enzyme leukocyte elastase (present in neutrophils), AAT deficiency leads to loss of the natural defense mechanisms due to lack of proper protease activities and results in inflammation that triggers emphysema, a common condition observed in COPD patients [24][26][27,29]. Significant genes connected with susceptibility towards COPD showed a nucleotide polymorphism (SNP) pattern with point mutations causing the replacement of a nucleotide with another in a particular gene locus, resulting in different alleles [27][30]. In a case-control cohort study conducted by Pillai et al. (2009), two SNPs were observed at the α-nicotinic acetylcholine receptor (CHRNA3/5 in chromosome 5) locus to be significant in lung dysfunction and increasing the risk for COPD (12.2% cases in the population presented this gene modification) [28][31]. Extensive cohort investigations of 1633–3000 individuals involving controls (smokers) and COPD patients were studied for SNPs and their pedigree analysis. The two SNPs (rs8034191 and rs1051730) in the CHRNA3/5 locus were found to be the most reliably associated with COPD and significantly associated with lung function or the FEV1 parameter [29][32]. Furthermore, the observation provided by Wilk et al., 2012, showed that CHRNA3/5 is a risk factor independent of smoking [30][33]. Next, the HHIP (Hedgehog interacting protein) locus in chromosome 4q31, a part of the hedgehog gene family, was involved in morphogenesis and lung development [28][29][30][31,32,33]. In the same region on chromosome 15q25.1, the IREB2 (Iron responsive element binding protein 2) and ADPHD1 (Aspartate beta-hydroxylase domain containing 1) were found to be involved in COPD development. The HTR4 (5-hydroxytryptamine receptor 4) gene was also responsible for FEV1/FVC changes. Another important locus was identified on chromosome 19q13, where the CYP2A6 (Cytochrome P450 family 2 subfamily A member 6) gene was significant in smoking populations [31][32][33][34,35,36]. The CYP2A6 gene controls the enzyme required for nicotine metabolism and is vital in smokers. A study by Bloom and colleagues also implicated the gene responsible for hypoxia, EGLN2 (Egl-9 family hypoxia inducible factor 2), playing a role in COPD; furthermore, CYP2A6 acts independently of the nicotine metabolism and hence can be responsible for COPD in both smokers and non-smokers [34][37]. Other genome-wide association studies and meta-analysis examinations have also shown the identification of 39 new loci (such as EEFSEC, DSP, MTCL1, and SFTPD) relating COPD to lung function, asthma, pulmonary fibrosis, lung composition (cells, tissues, and smooth muscles) and other comorbidity factors [35][36][38,39].Table 1.
Gene polymorphisms in COPD.
| Gene Identified | Location of Polymorphisms | Critical Effects | Reference |
|---|---|---|---|
| α-1-antitrypsin (AAT) | ATT*Z allele (Pi Z) homozygosity, single amino acid substitution causing base pair changes | low levels of AAT in serum, accumulation in hepatocytes leading to liver damage, neutrophil inactivity, emphysema | [23][24][25][26][26,27,28,29] |
| Alpha-nicotinic acetylcholine receptor | 2 SNPs (rs8034191 and rs1051730) at locus of CHRNA3/5 in chromosome 5 | lung dysfunction (deviations in FEV1 parameter) | [28][29][31,32] |
| HHIP (Hedgehog interacting protein) | chromosome 4q31 (HHIP mutations) |
developmental problems in the lung and abnormality during morphogenesis | [28][29][30][31,32,33] |
| IREB2 (Iron responsive element binding protein 2) | chromosome 15q25.1 (SNP rs7937) |
lung developmental changes and emphysema | [31][37][34,40] |
| ADPHD1 (Aspartate beta-hydroxylase domain containing 1) | chromosome 15q25.1 | airflow obstruction, AAT deficiency | [31][34] |
| HTR4 (5-hydroxytryptamine receptor 4) | chromosome 5q31-q33 | FEV1/FVC changes, airflow obstruction | [33][36] |
| CYP2A6 (Cytochrome P450 family 2 subfamily A member 6) | chromosome 19q13 | nicotine metabolism affected | [31][34][34,37] |
| EGLN2 (Egl-9 family hypoxia inducible factor 2) | chromosome 19q13.2 | hypoxia response destroyed | [34][37] |
3.2. Epigenetic Regulation (Methylation and Deacetylation)
DNA methylation is a reversible modification of DNA structure involving the transfer of a methyl group onto the C5 position of the cytosine, often as part of a CpG island or cluster [38][41]. DNA methylation is found to play a critical role in COPD development, and this epigenetic mechanism can be altered by cigarette smoking (Table 2) [39][42]. Lung macrophages substantially affect the polarization of innate and adaptive immunity and the recognition and elimination of bacteria. In this context, it was detected that several inflammatory/immune-related genes of lung macrophages, including HSH2D (Hematopoietic SH2 domain containing), SNX10 (Sorting nexin 10), CLIP4 (CAP-Gly domain containing linker protein family member 4), and TYKZ are 95 CpG loci with significant difference of methylation [40][43]. As the authors confirmed, this DNA methylation of selected gene loci in lung macrophages is associated with metabolic differences regionally in the lung. Next, mitochondrial transcription factor A (mtTFA) was remarkably decreased in the skeletal muscle of COPD patients, which was enhanced by cigarette smoke [41][44]. This phenomenon was positively correlated with the initiation and progression of COPD [41][44]. Interestingly, the methylation pattern changes can be affected by air pollution components like particulate matter (PM), ozone, nitrogen oxides, and polyaromatic hydrocarbons [42][45]. Twenty-seven differentially methylated regions (DMRs) in CpGs in NEGR1 (Neuronal growth regulator 1), ARID5A (AT-rich interaction domain 5 A), FOXl2 (Forkhead box 12), WDR46 (WD repeat domain 46), AKNA (AT-hook transcription factor), and SYTL2 (Synaptotagmin like 2) genes were correlated with prolonged exposure to PM10 and nitrogen dioxide [43][46]. Differentially methylated regions were detected in parenchymal fibroblasts in COPD, located in genes such as TMEM44 (Transmembrane protein 44), RPH3AL (Rabphilin 3 A like), WNT3A (Wnt family member 3 A), HLA-DP1 (Major histocompatibility complex, class II, DP beta 1), and HLA-DRB5 (Major histocompatibility complex, class II, DR beta 5) [44][47]. In addition, GWAS suggested that common SERPINA1 variants might influence COPD risk and associated lung function phenotypes [45][48]. Furthermore, hypermethylation of SERPINA1 in COPD patients was associated with tobacco addiction [46][49]. As a result, this epigenetic change can affect excessive mucus secretion and production, and goblet cell metaplasia can cause COPD. Histone acetylation is a reversible epigenetic change unequivocally associated with increasing the propensity for gene transcription [47][50]. Histone deacetylation and histone acetylation comprise two enzyme families (histone acetyltransferases (HATs) and histone deacetylases (HDACs) [48][51] and play an influential role in the occurrence of inflammation in COPD [49][52]. Studies proved the changeability of the acetylation/deacetylation balance toward acetylation in patients with COPD (Table 2) and resultant inflammation [50][53]. An increase in acetylated histone 4 was found in current smokers; conversely, ex-smokers with COPD showed an increase in histone 3. Cigarette smoking and oxidative stress are two significant features to inhibit inflammation in lung parenchyma and airways in COPD cases. Consequently, cigarette smoking elevated oxidant stress and promoted COPD glucocorticoid resistance during patients’ treatment. It was associated with higher HDAC2 activity [51][54]. Ding et al. reported Trichostatin A-an inhibitor of HDAC1/2-suppressing skeletal muscle atrophy and histomorphological alteration in COPD individuals [52][55]. H3K9 histone acetylation was high in the COPD-diseased human bronchial epithelial group [53][56].Table 2.
Gene methylation and histone acetylation in COPD.
| Epigenetic Mechanism | Altering Factors | Targets | Phenotype/Function in COPD Context | Reference |
|---|---|---|---|---|
| Methylation | cigarette smoking, air pollution |
HSH2D, SNX10, CLIP4, TYKZ | regulation of lung macrophage activity, maintaining lung metabolic balance | [40][43] |
| mtTFA | hypermethylation of the promoter is associated with the initiation and progression of COPD | [41][44] | ||
| NEGR1, ARID5A, FOXl2, WDR46, AKNA, SYTL2 | air pollution-dependent regulation of gene expression in Asians | [43][46] | ||
| HLX, SPON2 | alteration of functional gene expression in parenchymal fibroblasts | [54][57] | ||
| IREB2, PSMA4 | smoke-independent association of COPD with genetic variants in chromosome 15q25.1 | [55][58] | ||
| Acetylation | cigarette smoking, regulators of HDACs activity (Trichostatin A) |
Histones: H3K9, H3, H4 | increased levels are associated with inflammation, active gene transcription | [53][56] |
