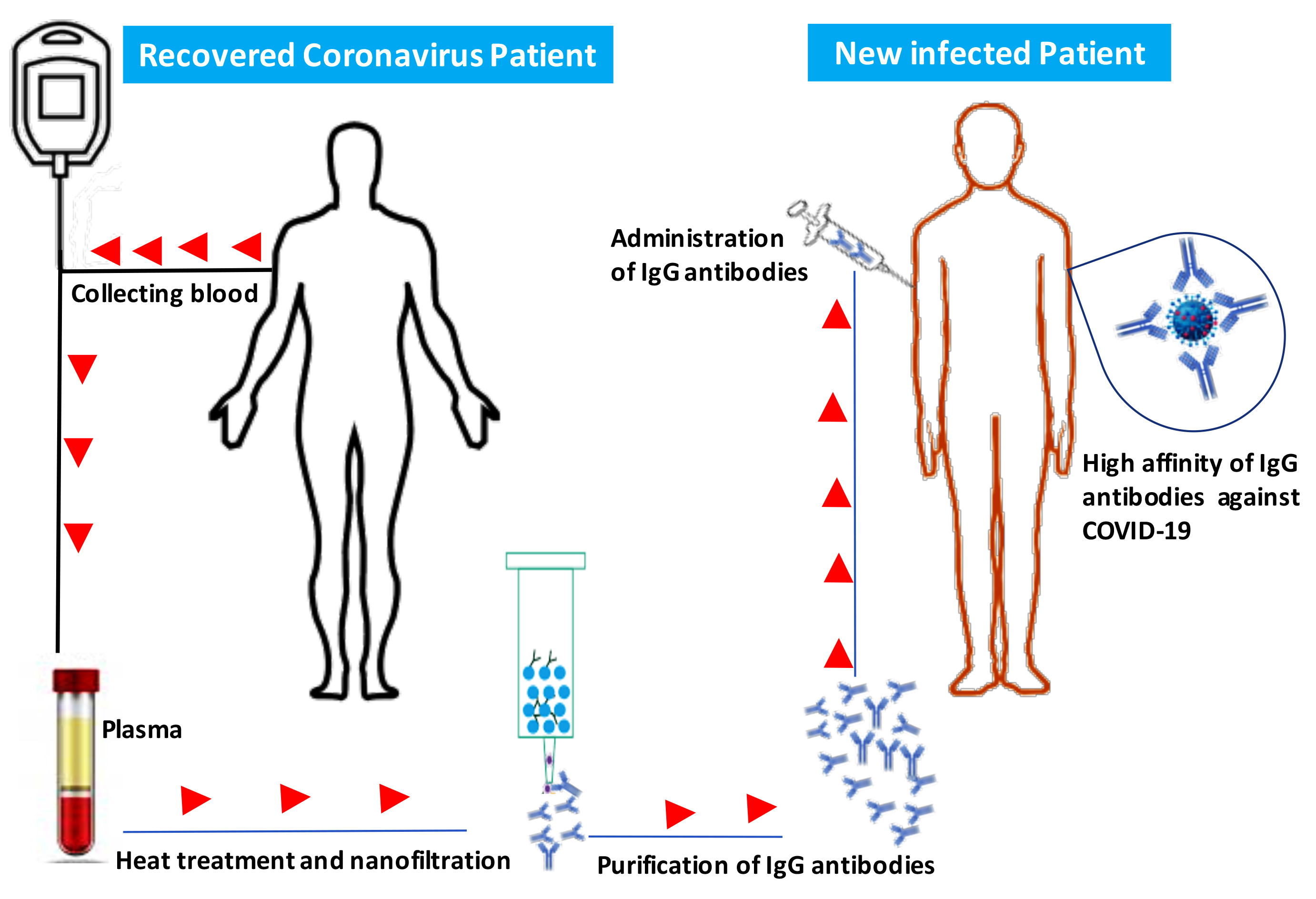
IThe emergG antibodies include two functional portions: the F(ab′)2 fragment, which is responsible for antigen recognition, and the crystallizable fragment (Fc), which is important for activation ofence of the novel coronavirus in Wuhan, China, which causes severe respiratory tract infections in humans (COVID-19), has become a global health concern. Most coronaviruses infect animals but can evolve into strains that cross the species barrier and infect humans. At the present, there is no single specific vaccine or efficient antiviral therapy against COVID-19. Recently, we showed that intravenous immunoglobulin (IVIg) treatment reduces inflammation of intestinal epithelial cells and eliminates overgrowth of the opportunistic human fungal pathogen Candida albicansin the murine gut. Immunotherapy with IVIg could be employed to neutralise COVID-19. However, the efficacy of IVIg would be better if the immune IgG antibodies were collected from the same city or the surrounding area of patients recovered from COVID-19 in order to increase the chance of neutralizing the virus. These immune IgG antibodies will be specific against COVID-19 by boosting the immune response by interacting with Fcγ receptors on B-cells and other innatin new infected patients. Different procedures may be used to remove or inactivate any possible pathogens from plasma of recovered Coronavirus patient-derived the immune cells. The Fc fragment also plays an important role in the activation of compleIgG, including solvent/detergent, 60°C heat-treatment, and nanofiltration. Overall, immunotherapy with immune IgG antibodies combined with antiviral drugs may be alternative treatment and in the clearance of microorganismsgainst COVID-19 until stronger options such as vaccines are available.
- COVID-19
- Coronavirus
- IgG
- IVIg
- Virus
- Immunotherapy
- SARS-CoV-2
1. Introduction
The emergence of the novel coronavirus in Wuhan, China, which causes severe respiratory tract infections in humans (COVID-19), has become a global health concern. Most coronaviruses infect animals but can evolve into strains that can also infect humans. Recently, we showed that intravenous immunoglobulin (IVIg) treatment reduces inflammation of intestinal epithelial cells and eliminates overgrowth of the opportunistic human fungal pathogen Candida albicans in the murine gut in association with downregulation of proinflammatory mediators combined with upregulation of anti-inflammatory cytokines [1].
Coronaviruses are enveloped positive-stranded RNA viruses belonging to the family Coronaviridae [2]. An envelope-anchored spike protein promotes coronavirus entry into host cells by first binding to a host receptor and then fusing viral and host membranes [2]. Whole-genome sequencing of viral RNA has revealed that the virus causing COVID-19 is phylogenetically related to the SARS-related coronaviruses first isolated in Chinese horseshoe bats during 2015‒2017 [3][4]. Researchers in Guangzhou, China, have recently suggested that pangolins are the probable animal source of the COVID-19 outbreak [5]. In terms of the interaction between the virus and its host, Lu et al. have reported that angiotensin-converting enzyme 2 (ACE 2) is most probably used by the spike protein of the SARS-CoV-2OVID-19 virus as a receptor similar to that SARS-CoV [6].
Recently, Tang et al. showed that the SARS-CoV-2OVID-19 has evolved into two major lineages—dubbed ‘L’ and ‘S’ types. The older ‘S-type’ appears to be milder and less infectious, while the ‘L-type’, which emerged later, spreads quickly and is currently more aggressive than the S-type [7]. Current symptoms reported for patients with COVID-19 have included mild to severe respiratory illness with fever, fatigue, cough, myalgia, and difficulty breathing [8]. Tyrrell et al. showed that infected respiratory epithelial cells by coronavirus become vacuolated and show damaged cilia that lead to production of inflammatory mediators, which increase nasal secretion and cause local inflammation and swelling [9]. These responses in turn stimulate sneezing, obstruct the airway, and raise the temperature of the mucosa [9].
Currently, there is no single specific vaccine or effective antiviral therapy against SARS-CoV-2OVID-19. Several pharmaceutical and biotechnological companies are working on vaccine development and estimate that this vaccine will take years to develop and test before it can reach a large population. Additionally, there are currently no approved treatments for any coronavirus disease, including COVID-19. Several antiviral drugs are being tested, and initial findings are expected soon. Individuals with weakened immune systems appear to be at greater risk of developing complications associated with COVID-19. Immunotherapy using IgG in combination with antiviral drugs could be used to treat or prevent COVID-19 and to strengthen our immune response against this virus [10][11]. IgG antibodies include two functional portions: the F(ab′)2 fragment, which is responsible for antigen recognition, and the crystallizable fragment (Fc), which is important for activation of the immune response by interacting with Fcγ receptors on B-cells and other innate immune cells [12]. The Fc fragment also plays an important role in the activation of complement and in the clearance of microorganisms [12].
2. Description
IVIg is a pool of IgG from thousands of healthy donors, and exposure of individual donors to endemic infectious diseases, vaccines, and ubiquitous microorganisms participates in the production of IgG antibodies against different microorganisms and their products [13][14][15].
IVIg has been used to treat patients with autoimmune and chronic inflammatory diseases, such as dermatomyositis, Kawasaki disease, multiple sclerosis, lupus, chronic lymphocytic leukemia, and idiopathic thrombocytopenic purpura [16][17][18]. Furthermore, IVIg has also been used as an anti-infectious agent against viruses, bacteria, and fungi in human patients and experimental models [13][19][20][21]. IVIg treatment may result in some adverse events, which are associated with specific immunoglobulin preparations and individual differences, but many clinical and experimental studies show that switching from IVIg to subcutaneous immunoglobulin can minimize these adverse events [22][23][24].
IVIg plays an important role in the prevention of infectious episodes in primary immunodeficient patients, and the beneficial effects of these antibodies in the treatment of infectious diseases goes beyond simple neutralization of microorganisms or their toxins. Anti-inflammatory pathways are also critical for protection against infection [25].
IVIg may modulate the immune response via multiple mechanisms, including blocking a wide array of proinflammatory cytokines, Fc-gamma receptors (FcγRs), and leukocyte adhesion molecules, suppressing pathogenic Th1 and Th17 subsets, and neutralizing pathogenic autoantibodies [26][27][28]. IVIg can also expand regulatory T-cells by induction of cyclo-oxygenase-2-dependent prostaglandin E2 production in dendritic cells [29].
References
- Rogatien Charlet; Boualem Sendid; Srini V Kaveri; Daniel Poulain; Jagadeesh Bayry; Samir Jawhara; Intravenous Immunoglobulin Therapy Eliminates Candidaalbicans and Maintains Intestinal Homeostasis in a Murine Model of Dextran Sulfate Sodium-Induced Colitis.. International Journal of Molecular Sciences 2019, 20, 1473, 10.3390/ijms20061473.
- Fang Li; Structure, Function, and Evolution of Coronavirus Spike Proteins. Annual Review of Virology 2016, 3, 237-261, 10.1146/annurev-virology-110615-042301.
- Dan Hu; Changqiang Zhu; Lele Ai; Ting He; Yi Wang; Fuqiang Ye; Lu Yang; Chenxi Ding; Xuhui Zhu; Ruicheng Lv; et al.Jin ZhuBachar HassanYouJun FengWeilong TanChangjun Wang Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats.. Emerging Microbes & Infections 2018, 7, 154-10, 10.1038/s41426-018-0155-5.
- Jasper Fuk-Woo Chan; Shuofeng Yuan; Kin-Hang Kok; Kelvin Kai-Wang To; Hin Chu; Jin Yang; Fanfan Xing; Jieling Liu; Cyril Chik-Yan Yip; Rosana Wing-Shan Poon; et al.Hoi-Wah TsoiSimon Kam-Fai LoKwok-Hung ChanVincent Kwok-Man PoonWan-Mui ChanJonathan Daniel IpJian-Piao CaiVincent Chi-Chung ChengHonglin ChenChristopher Kim-Ming HuiKwok-Yung Yuen A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet 2020, 395, 514-523, 10.1016/s0140-6736(20)30154-9.
- Liu, P.; Chen, W.; Chen, J.P.; Viral Metagenomics Revealed Sendai Virus and Coronavirus Infection of Malayan Pangolins (Manis javanica).. Viruses 2019, 11, 979, doi.org/10.3390/v11110979.
- Roujian Lu; Xiang Zhao; Juan Li; Peihua Niu; Bo Yang; Honglong Wu; Wenling Wang; Hao Song; Baoying Huang; Na Zhu; et al.Yuhai BiXuejun MaFaxian ZhanLiang WangTao HuHong ZhouZhenhong HuWeimin ZhouLi ZhaoJing ChenYao MengJi WangYang LinJianying YuanZhihao XieJinmin MaWilliam J LiuDayan WangWenbo XuEdward C HolmesGeorge F GaoGuizhen WuWeijun ChenWeifeng ShiWenjie Tan Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet 2020, 395, 565-574, 10.1016/s0140-6736(20)30251-8.
- Xiaolu Tang; Changcheng Wu; Xiang Li; Yuhe Song; Xinmin Yao; Xinkai Wu; YuanGe Duan; Hong Zhang; Yirong Wang; Zhaohui Qian; et al.Jie CuiJian Lu On the origin and continuing evolution of SARS-CoV-2. National Science Review 2020, nwaa036, 36, 10.1093/nsr/nwaa036.
- Sijia Tian; Nan Hu; Jing Lou; Kun Chen; Xuqin Kang; Zhenjun Xiang; Hui Chen; Dali Wang; Ning Liu; Dong Liu; et al.Gang ChenYongliang ZhangDou LiJianren LiHuixin LianShengmei NiuLuxi ZhangJinjun Zhang Characteristics of COVID-19 infection in Beijing.. Journal of Infection 2020, 80, 401-406, 10.1016/j.jinf.2020.02.018.
- Tyrrell, D.A.J.; Myint, S.H.. Coronaviruses; Baron, S., Ed.; Galveston (TX), Eds.; Galveston, Tex: University of Texas Medical Branch at Galveston: New York, NY, USA, 1996; pp. ..
- Pyrc, K.; Bosch, B.J.; Berkhout, B.; Jebbink, M.F.; Dijkman, R.; Rottier, P.; van der Hoek, L.; Inhibition of human coronavirus NL63 infection at early stages of the replication cycle. Antimicrob.. Agents Chemother. 2006, 50, 2000–2008.
- Sangeetha Rao; William Sasser; Franco Díaz; Nirmal Sharma; Jeffrey Alten; Coronavirus Associated Fulminant Myocarditis Successfully Treated With Intravenous Immunoglobulin and Extracorporeal Membrane Oxygenation. Chest 2014, 146, 336A, 10.1378/chest.1992018.
- Caroline Galeotti; Srini V Kaveri; Jagadeesh Bayry; IVIG-mediated effector functions in autoimmune and inflammatory diseases. International Immunology 2017, 29, 491-498, 10.1093/intimm/dxx039.
- Binh An Diep; Vien T. M. Le; Cédric Badiou; Hoan N. Le; Marcos Gabriel Pinheiro; Au H. Duong; Xing Wang; Etyene Castro Dip; Fábio Aguiar-Alves; Li Basuino; et al.Helene MarbachThuy T. MaiMarie N. SardaOsamu KajikawaGustavo Matute-BelloChristine TkaczykJean-Philippe RasigadeBret R. SellmanHenry F. ChambersGerard Lina IVIG-mediated protection against necrotizing pneumonia caused by MRSA. Science Translational Medicine 2016, 8, 357ra124-357ra124, 10.1126/scitranslmed.aag1153.
- Valérie Gauduchon; Nathalie Eyssade; Simone Peyrol; Gregoire Cozon; François Vandenesch; Anne-Laure Genestier; J Etienne; Gerard Lina; Neutralization of Staphylococcus aureus Panton Valentine Leukocidin by Intravenous Immunoglobulin In Vitro. The Journal of Infectious Diseases 2004, 189, 346-353, 10.1086/380909.
- I. Krause; R. Wu; Y. Sherer; M. Patanik; J. B. Peter; Y. Shoenfeld; In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations - a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases*. Transfusion Medicine 2002, 12, 133-139, 10.1046/j.1365-3148.2002.00360.x.
- Jolles, S.; Sewell, W.A.; Misbah, S.A.; Clinical uses of intravenous immunoglobulin in pregnancy. American Journal of Obstetrics and Gynecology 1997, 177, 1560-1561, 10.1016/s0002-9378(97)70120-0.
- Srini V Kaveri; Mohan S. Maddur; Pushpa Hegde; S. Lacroix-Desmazes; Jagadeesh Bayry; Intravenous immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clinical & Experimental Immunology 2011, 164, 2-5, 10.1111/j.1365-2249.2011.04387.x.
- Margot Samson; William Fraser; David Lebowitz; Treatments for Primary Immune Thrombocytopenia: A Review.. Cureus 2019, 11, e5849, 10.7759/cureus.5849.
- Bayry, J.; Lacroix-Desmazes, S.; Kazatchkine, M.D.; Kaveri, S.V.; Intravenous immunoglobulin for infectious diseases: Back to the pre-antibiotic and passive prophylaxis era?. Trends Pharm. Sci. 2004, 25, 306–310.
- Bo Shopsin; Srini V Kaveri; Jagadeesh Bayry; Tackling Difficult Staphylococcus aureus Infections: Antibodies Show the Way.. Cell Host & Microbe 2016, 20, 555-557, 10.1016/j.chom.2016.10.018.
- David Ben Nathan; Shlomo Lustig; Guy Tam; Shahar Robinzon; Shraga Segal; Bracha Rager-Zisman; Prophylactic and Therapeutic Efficacy of Human Intravenous Immunoglobulin in Treating West Nile Virus Infection in Mice. The Journal of Infectious Diseases 2003, 188, 5-12, 10.1086/376870.
- Hans D. Ochs; Sudhir Gupta; Peter Kiessling; Uwe Nicolay; Melvin Berger; the Subcutaneous IgG Study Group; Safety and Efficacy of Self-Administered Subcutaneous Immunoglobulin in Patients with Primary Immunodeficiency Diseases. Journal of Clinical Immunology 2006, 26, 265-273, 10.1007/s10875-006-9021-7.
- Lars Høj Markvardsen; Jean-Christophe Debost; Thomas Harbo; Søren Sindrup; H. Andersen; I. Christiansen; M. Otto; N. K. Olsen; L. L. Lassen; J. Jakobsen; et al.The Danish CIDP and MMN Study Group Subcutaneous immunoglobulin in responders to intravenous therapy with chronic inflammatory demyelinating polyradiculoneuropathy. European Journal of Neurology 2013, 20, 836-842, 10.1111/ene.12080.
- Thomas Harbo; Henning Andersen; Johannes Jakobsen; Long-term therapy with high doses of subcutaneous immunoglobulin in multifocal motor neuropathy. Neurology 2010, 75, 1377-1380, 10.1212/wnl.0b013e3181f735ce.
- Ruchi Srivastava; Chandran Ramakrishna; Edouard M. Cantin; Anti-inflammatory activity of intravenous immunoglobulins protects against West Nile virus encephalitis. Journal of General Virology 2015, 96, 1347-1357, 10.1099/vir.0.000079.
- Seite, J.F.; Shoenfeld, Y.; Youinou, P.; Hillion, S.; What is the contents of the magic draft IVIg?. Autoimmun. Rev. 2008, 7, 435–439.
- Mohan S Maddur; Jamma Trinath; Magalie Rabin; Francis Bolgert; Moneger Guy; Jean-Michel Vallat; Laurent Magy; Kithiganahalli N Balaji; Srini V Kaveri; Jagadeesh Bayry; et al. Intravenous immunoglobulin-mediated expansion of regulatory T cells in autoimmune patients is associated with increased prostaglandin E2 levels in the circulation. Cellular & Molecular Immunology 2014, 12, 650-652, 10.1038/cmi.2014.117.
- Mohan S. Maddur; Magalie Rabin; Pushpa Hegde; Francis Bolgert; Moneger Guy; Jean-Michel Vallat; Laurent Magy; Jagadeesh Bayry; Srini V Kaveri; Intravenous immunoglobulin exerts reciprocal regulation of Th1/Th17 cells and regulatory T cells in Guillain–Barré syndrome patients. Immunologic Research 2014, 60, 320-329, 10.1007/s12026-014-8580-6.
- Jamma Trinath; Pushpa Hegde; Meenu Sharma; Mohan S. Maddur; Magalie Rabin; Jean-Michel Vallat; Laurent Magy; Kithiganahalli N. Balaji; Srini V Kaveri; Jagadeesh Bayry; et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2–dependent prostaglandin E2 in human dendritic cells. Blood 2013, 122, 1419-1427, 10.1182/blood-2012-11-468264.