Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Rafael Resende Assis Silva and Version 2 by Conner Chen.
Sustainable and biodegradable bioplastics are gaining significant attention due to resource depletion and plastic pollution. An increasing number of environmentally friendly plastics are being introduced to the market with the aim of addressing these concerns. However, many final products still contain additives or mix non-biodegradable polymers to ensure minimum performance, which often undermines their ecological footprint. Moreover, there is a lack of knowledge about all stages of biodegradation and their accuracy in classifying products as biodegradable.
- biodegradation
- biodegradable plastics
- biodegradable polymers
1. Mechanisms of Degradation
Polymer degradation refers to any chemical, physical, or biochemical reaction that involves breaking covalent bonds in the backbone of the polymer, resulting in an irreversible change in its properties due to alterations in the chemical structure and the reduction of molecular weight. The breaking of primary chemical bonds in the main or side chain generates reactive species (free radicals) that are responsible for propagating the degradation process of the polymeric artifact. The initiation of the polymer degradation process is catalyzed by abiotic factors, e.g., heat, light, radiation, humidity, pH of the medium, mechanical stress, and chemical attack. These forms of initiation require activation energy for breaking chemical bonds in the polymer, with the binding energy varying according to the atoms’ connection, i.e., they can have ionic, coordinate, metallic, or covalent primary bonds. Generally, the types of bonds in organic polymers are covalent and usually involve short distances and high energies (1.5 Å and 100 K/mol) [1][13]. The main covalent bonds found in organic polymers, their binding energy, stability, and binding distance, are discussed in-depth by Canevarolo (2006) [1][13].
Chain scission or bond breaking occurs when the localized energy in this chemical bond is greater than the energy of the bond. When a more unstable bond is positioned in side groups or short branches, its breakage leads to (i) the loss of that side group or (ii) its modification by the insertion of new atoms (e.g., oxygen), resulting in polymer degradation. This type of degradation can occur both in the solid and molten states. The energy required for bond scission can be provided in different ways, such as heat (thermolysis), water (hydrolysis), oxygen (oxidation), chemistry (solvolysis), light (photolysis), gamma radiation (radiolysis), or shear (mechanical) or weathering (generally UV/ozone degradation), etc.
1.1. Hydrolysis
Hydrolysis is a chemical decomposition process that involves breaking a bond by reacting with water molecules. The hydrolysis process is the most important for initiating the biodegradation of synthetic polymers, especially polyesters. The rate of hydrolytic degradation varies from a few hours to years, depending mainly on the degree of crystallinity, type of functional group, molecular weight, main skeletal structure, morphology, temperature, and pH of the medium. According to Lyu & Untereker (2009) [2][14], hydrolytic degradation is divided into three levels. The first level involves degradation at the molecular level, in which hydrolysis is controlled only by chemical reactivity. The second level is also molecular but is associated with molecular mobility and water–polymer interactions. The third level is the macroscopic one, where erosion and water diffusion reaction are the governing parameters for degradation. Therefore, hydrolysis can cause biopolymers to degrade either through surface erosion or bulk erosion. During surface erosion, the outer layer of the polymer degrades first, while the inner material is degraded last. In contrast, bulk erosion occurs when water molecules quickly diffuse into the amorphous regions of the polymer, causing a rapid loss of strength and structural properties [3][12]. Hydrolysis occurs mainly in hygroscopic polymers and those with water-sensitive groups in the polymeric backbone. During hydrolysis, the polymer is always split into two components; otherwise, it will not be considered hydrolysis (hydro = water; lysis = breakdown). If the products are not ionized, one part gains a hydrogen atom (H+), and the other gains a hydroxyl group (OH-) from the broken water molecule. Figure 12 shows the hydrolysis rate ranking of the main polymers that undergo degradation when exposed to moisture, e.g., polyanhydrides, polyesters, polyethers, polyamides, polycarbonates, etc. Furthermore, it is shown that hydrolysis also depends on the polymer’s polarity and degree of crystallinity. More hydrophobic polymers have a lower reaction rate because the water content in the polymer and the water permeability decrease with decreasing polymer polarity. Therefore, hydrolytic stability increases in the same order as hydrophobicity. In turn, an increase in crystalline phases in polymers inhibits the plasticization of the polymer by the water in these regions since the steric effect and strong intermolecular interactions impede water penetration in the ordered regions, i.e., crystalline.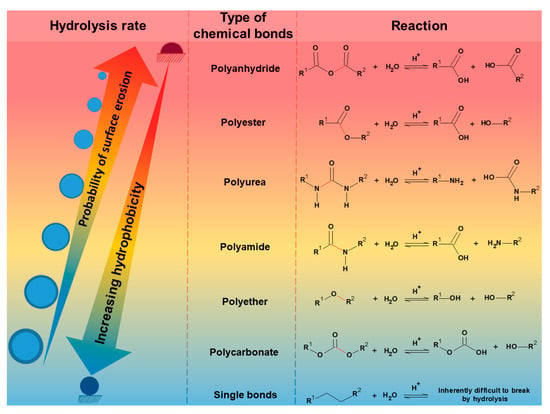
Figure 12.
Ranking of the hydrolysis rate of the main polymers that suffer degradation when exposed to humidity.
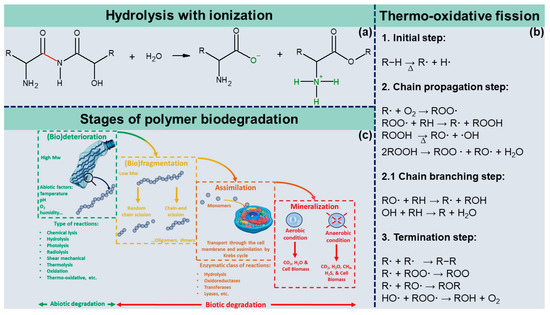
Figure 23. Ionization of an amino acid after hydrolysis (a); steps of thermo-oxidative degradation reactions (b); stages of polymer biodegradation (c).
1.2. Thermolysis
Thermal decomposition, also known as thermolysis, is a chemical reaction in which a reacting substance decomposes into at least two new substances upon heating. In the case of polymers, thermal decomposition generates molecules and atoms that are different from the precursor without the simultaneous involvement of other reagents such as oxygen. Since the heat received breaks the bonds of the molecules of the reactants, thermal decomposition is generally an endothermic process. If the chemical energy of the reactants is greater than that of the products, the decomposition reaction will be exothermic (ΔH), indicating that the reactants are highly reactive and the products are stable. An exothermic decomposition reaction releases heat and may be accompanied by an explosion or another chemical reaction. The results should be conceptualized with the term “decomposition temperature” instead of “degradation temperature”. Unfortunately, the latter term is treated as a synonym for the former, which is incorrect. Degradation temperature refers to the temperature at which loss of some function or property of the material being studied occurs. For example, protein denaturation, inactivation of active antimicrobial agents, change in color or transparency, and reduction in mechanical or barrier performance to gases. On the other hand, the decomposition temperature (TDT) should be used to discuss TGA results because it refers to the decomposition of the polymer into smaller molecules, constituent atoms, and/or the release of gases such as CO2, CH4, CO, etc. In this sense, the degradation temperature often occurs before the decomposition temperature because most properties and functions of materials are thermosensitive, and some properties depend on secondary (intermolecular) bonds that break at mild temperatures. Therefore, when the TGA detects mass loss, it is crucial to describe the event as thermal decomposition as it necessarily involves the breaking of primary bonds, confirming the occurrence of material thermolysis.1.3. Oxidation and Thermo-Oxidative Fission
In oxidation reactions, a reduction in the average molar mass of the polymer is not necessarily observed, but a marked change in its physical and chemical properties, e.g., a color change of the material, may occur. Regardless of the atmosphere’s composition, polymers will start to decompose if heated enough. However, thermal oxidation differs from thermal decomposition in that it generally catalyzes oxidation reactions culminating in material decomposition at milder temperatures. Thermo-oxidative fission of polymers is a self-catalytic process that occurs in three stages: initiation, propagation, and termination. The oxygen molecule is considered a highly reactive chemical species, as it reacts quickly with any environmental free radicals. In the first step, heat-catalyzed degradation is initiated when polymer chains form radicals (R*) either by hydrogen abstraction or by homolytic scission of the C-C bond. Next, the propagation of degradation involves a series of intermediate reactions. The first intermediate step is the reaction of a free radical (R·) with an oxygen molecule (O2), forming a peroxy radical (ROO·) that abstracts a hydrogen atom from another polymeric chain, producing a hydroperoxide (ROOH). Hydroperoxides are highly unstable; therefore, they decompose into two new free radicals, (RO·) + (·OH), which attack the polymer chain, abstracting labile hydrogens and introducing new radicals [6][17]. The thermo-oxidative reaction ends by recombining two radicals, forming stable products, or abstracting hydrogen or π bonds. Figure 23b shows the thermo-oxidative degradation reactions elucidated above.2. Abiotic and Biotic Degradation
The degradation process of a polymer depends on its intrinsic properties and the extrinsic conditions to which it is exposed, such as the biodiversity and occurrence of microorganisms, which vary locally. Therefore, the degradation of materials can generally be classified as abiotic (heat, radiation, oxygen, humidity, solvents/chemicals) or biotic (bacteria, fungi, algae). Abiotic degradation is usually the first stage after the end of the useful life of the plastic, during which physical and chemical changes occur, but not biological actions, resulting in the modification of at least one property or characteristic of the material. Some of these alterations are visible to the naked eye, such as changes in color, dimensions, cracks, and weight, while others require tools for characterization, such as mechanical and rheological properties, degree of crystallinity, oxidation state, and molecular weight distribution. In nature, biotic and abiotic factors can act together to decompose organic matter. This is because some microbes excrete extracellular enzymes that act directly on plastics, and prior fragmentation and reduction of molar mass are not necessary to make the microorganisms available. An example of this is the degradation of polyhydroxybutyrate (PHB) by the action of intracellular and extracellular depolymerase of bacteria and fungi [7][18]. However, abiotic factors weaken the polymer structure, producing smaller polymer fragments that can pass through cell membranes and are biodegraded within microbial cells by cellular enzymes, catalyzing the biological stage of biodegradation. Most plastics degrade first at the polymer surface, as it is the most exposed and vulnerable to chemical (abiotic) or bacterial/enzyme (biotic) attack. The Table 1 presents a list of enzymes and bacteria involved in the biodegradation of various types of polymers, including the type of polymer, biodegradation mechanism, mode of action and mechanisms.Table 1.
Enzymes and bacteria involved in biodegradation of polymers.
| Type of Enzyme/Bacteria | Polymer Type | Biodegradation Mechanism | Mode of Action and Mechanisms |
|---|---|---|---|
| Proteases | Proteins | Hydrolysis | Catalyze the cleavage of peptide bonds in proteins, breaking them down into smaller peptides and eventually amino acids. |
| Lipases | Lipids | Hydrolysis | Break down ester bonds in lipids, producing free fatty acids and glycerol. |
| Amylases | Starch | Hydrolysis | Break down the α-1,4-glycosidic bonds in starch, producing glucose. |
| Cellulases | Cellulose | Hydrolysis | Break down the β-1,4-glycosidic bonds in cellulose, producing glucose. |
| Chitinases | Chitin | Hydrolysis | Break down the β-1,4-glycosidic bonds in chitin, producing N-acetylglucosamine. |
| Laccases | Lignin | Oxidation | Oxidize the phenolic and non-phenolic structures in lignin, breaking down the polymer into smaller fragments. |
| Peroxidases | Lignin | Oxidation | Catalyze the oxidation of lignin by hydrogen peroxide or oxygen, breaking it down into smaller fragments. |
| Kosakonia sp. | Polyethylene | Anaerobic metabolism | Production of extracellular enzymes to break down polyethylene into smaller fragments for cellular uptake and utilization as carbon and energy sources. |
| Aspergillus sp. | Various | Aerobic metabolism | Produce reactive oxygen species and a range of extracellular enzymes, e.g., cellulases, hemicellulases, and ligninases. |
The data in the table were constructed based on references [7][8][9][18,19,20].
On the other hand, biotic degradation is classified as the biodegradation caused by the action of microorganisms that modify and consume the polymer or polymeric monomers, producing molecules of low molar mass (acids, aldehydes, terpenes, and H2O) and gases (CO2, CH4, and N2). According to Oliveira et al. (2020), the main biodegradation mechanism is the adhesion of microorganisms to the polymer surface, followed by the colonization of the exposed surface. After colonization, enzymatic degradation of the polymer occurs by hydrolytic cleavage, producing molecules of low molecular weight until the final mineralization in CO2 and H2O [10][11][21,22].
