Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yudong Zhang and Version 2 by Conner Chen.
Alzheimer’s and related diseases are significant health issues of this era. The interdisciplinary use of deep learning in this field has shown great promise and gathered considerable interest.
- deep learning
- Alzheimer’s disease
- mild cognitive impairment
1. Alzheimer’s Disease and Mild Cognitive Impairment
Alzheimer’s disease is the most common form of dementia and a significant health issue of this era [1][4]. Brookmeyer et al. [2][5] predicted that more than 1% of the world population would be affected by AD or related diseases by 2050, with a significant proportion of this cohort requiring a high level of care. AD usually starts from middle-to-old age as a chronic neurodegenerative disorder, but rare cases of early-onset AD can affect individuals of 45–64 years old [3][6]. AD leads to cognitive decline symptoms: memory impairment [4][7], language dysfunction [5][8], and decline in cognition and judgment [6][9]. An individual with symptoms may require moderate to constant assistance in day-to-day life, depending on the stage of disease progression. These symptoms severely affect patients’ quality of life (QOL) and their families. Studies into cost-of-illness for dementia and AD reveal that the higher societal need for elderly care significantly increases overall social-economic pressure [7][10].
The biological process that leads to AD may begin more than 20 years before symptoms appear [8][11]. The current understanding of AD pathogenesis is based on amyloid peptide deposition and the accumulation and phosphorylation of tau proteins around neurons [9][10][11][12,13,14], which leads to neurodegeneration and eventual brain atrophy. Factors associated with AD include age, genetic predisposition [12][15], Down’s syndrome [13][16], brain injuries [14][17], and cardiorespiratory fitness [15][16][17][18,19,20]. AD-related cognitive impairment can be broadly separated into three stages: (1) preclinical AD, where measurable changes in the brain, cerebral spinal fluid (CSF), and blood plasma can be detected; (2) mild cognitive impairment (MCI) due to AD, where biomarker evidence of AD-related brain change can be present; and (3) dementia due to AD, where changes in the brain are evident and noticeable memory, thinking and behavioural changes appear and impair an individual’s daily function.
The condition most commonly associated with AD is mild cognitive impairment (MCI), the pre-dementia stage of cognitive impairment. However, not all cases of MCI develop into AD. Since no definite pathological description exists, MCI is currently perceived as the level of cognitive impairment above natural age-related cognitive decline [18][19][21,22]. Multiple studies have analyzed the demographics and progression of MCI and have found the following: 15–20% of people age 65 or older have MCI from a range of possible causes [20][23]; 15% of people age 65 or older with MCI developed dementia at two years follow-up [21][24]; and 32% developed AD and 38% developed dementia at five years follow-up [22][23][25,26]. The early diagnosis of MCI and its subtypes can lead to early intervention, which can profoundly impact patient longevity and QOL [24][27]. Therefore, better understanding the condition and developing effective and accurate diagnostic methods is of great public interest.
2. Diagnostic Methods and Criteria
The current standard diagnosis of AD and MCI is based on a combination of various methods. These methods include cognitive assessments such as the Mini-Mental State Examination [25][26][27][28,29,30], Clinical Dementia Rating [28][29][31,32], and Cambridge Cognitive Examination [30][31][33,34]. These exams usually take the form of a series of questions and are often performed with physical and neurological examinations. Medical and family history, including psychiatric history and history of cognitive and behavioral changes, are also considered in the diagnosis. Genetic sequencing for particular biomarkers, such as the APOE-e4 allele [32][35], is used to determine genetic predisposition.
Neuroimaging is commonly used to inspect various signs of brain changes and exclude other potential causes. Structural magnetic resonance and diffusion tensor imaging are widely applied to check for evidence of symptoms of brain atrophy. Various forms of computed tomography (CT) are also used in AD and MCI diagnosis. Regarding positron emission tomography (PET), FDG-PET [33][36] inspects brain glucose metabolism, while amyloid-PET is applied to measure beta-amyloid levels. Single-photon emission computed tomography [34][37] (SPECT) is likely to produce false-positive results and is inadequate in clinical use. However, SPECT variants can be potentially used in diagnosis, e.g., 99 mTc-HMPAO SPECT [35][36][38,39]. At the same time, FP-CIT SPECT can visualize discrepancies in the nigrostriatal dopaminergic neurons [37][40]. In neuroimaging, a combination of multiple modalities is commonly used to utilize the functionality of each modality.
New diagnostic factors of CSF and blood plasma biomarkers have been reported in the literature and have been deployed in clinical practice in recent years. There are three main CSF and blood plasma biomarkers: Amyloid-β42, t-tau, and p-tau. Other biomarkers include neurofilament light protein (NFL), and neuron-specific enolase (NSE, and HFABP [38][39][41,42]. CSF biomarkers are becoming a critical factor in AD diagnostic criteria in some practices. However, the actual ‘ground truth’ diagnosis of AD can only be made via post-mortem autopsy.
Before this century, the established diagnostic criteria were the NINCDS-ADRDA criteria [40][41][43,44]. These criteria were updated by the International Working Group (IWG) in 2007 to include requirements of at least one factor among MRI, PET, and CSF biomarkers [42][45]. A second revision was introduced in 2010 to include both pre-dementia and dementia phases [43][46]. This was followed by a third revision to include atypical prodromal Alzheimer’s disease that shows cognition deficits other than memory impairment—IWG-2 [44][47]. Another independent set of criteria, the NIA-AA criteria, was introduced in 2011. These criteria include measures of brain amyloid, neuronal injury, and degeneration [45][48]. Individual criteria were introduced for each clinical stage, including pre-clinical [46][47][49,50], MCI [48][49][51,52], dementia [50][51][53,54], and post-mortem autopsy [52][55].
3. The Deep Learning Approach
Detailed preprocessing with refined extraction of biomarkers combined with statistical analysis is the accepted practice in current medical research. Risacher et al. [53][56] applied statistical analysis on biomarkers extracted using voxel-based morphometry and parcellation methods from T1-weighted MRI scans of AD, MCI, and HC. The study reveals statistical significance in multiple measures, including hippocampal volume and entorhinal cortex thickness. Qiu et al. [54][57] further confirmed this significance by analyzing regional volumetric changes through large deformation diffeomorphic metric mapping (LDDMM). Guevremont et al. [55][58] focused on robustly detecting microRNAs in plasma and used standardized analysis to identify microRNA biomarkers in different phases of Alzheimer’s disease. This study and its statistical analysis yielded useful diagnostic markers reflecting the underlying disease pathology. The different biomarker information extracted was fed into statistical analysis methods with varying numbers of variables to detect changes in biomarkers in disease development [56][59]. Similar studies also employed other neuroimaging data, genetic data, and CSF biomarkers. These studies supported the use of MRI imaging biomarkers in AD [57][60] and MCI diagnosis [58][61], laying the basis for developing automatic diagnostic algorithms.
Machine learning has amassed great popularity among current automated diagnostic algorithms due to its adaptivity to data and the ability to generalize knowledge with lower requirements of expert experience. The study by Klöppel et al. [59][62] proved the validity of applying machine learning algorithms in diagnosing dementia through a performance comparison between the Support Vector Machine (SVM) classification of local grey matter volumes and human diagnosis by professional radiologists. Janousova et al. [60][63] proposed penalized regression with resampling to search for discriminative regions to aid Gaussian kernel SVM classification. The regions found by the study coincide with the previous morphological studies. These breakthroughs led to the development of many machine-learning algorithms for AD and MCI detection. Zhang et al. [61][64] proposed a kernel combination method for the fusion of heterogeneous biomarkers for classification with linear SVM. Liu et al. [62][65] proposed the Multifold Bayesian Kernelization (MBK) algorithm, where a Bayesian framework derives kernel weights and synthesis analysis provides the diagnostic probabilities of each biomarker. Zhang et al. [63][66] proposed the extraction of the eigenbrain using Welch’s t-test (WTT) [64][67] combined with a polynomial kernel SVM [65][68] and particle swarm optimization (PSO) [66][69].
There has also been considerable interest in applying deep learning (DL), a branch of machine learning, to the field of AD and related diseases. Deep learning integrates the two-step feature extraction and classification process into neural networks, universal approximators based on backpropagation parameter training. [67][70]. Deep learning has made considerable advances in the domain of medical data, e.g., breast cancer [68][71], tuberculosis [69][72], and glioma [70][73]. Instead of hand-crafting features, models, and optimizers, deep learning leverages the layered structure of neural networks for the automated abstraction of various levels of features. For example, Feng et al. [71][74] used the proposed deep learning model to extract biomarkers for MRI in neuroimaging. The study demonstrates that the deep learning approach outperformed other neuroimaging biomarkers of amyloid and tau pathology and neurodegeneration in prodromal AD. A visualization of the field of this survey is shown in Figure 1.
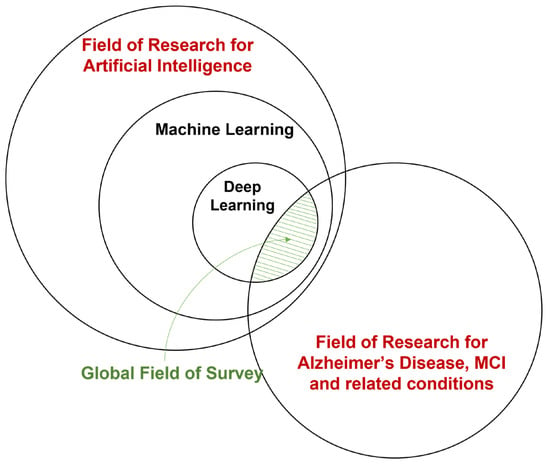
Figure 1.
A broad overview of the field of this survey.
4. Areas of Interest
The primary aim of the surveyed deep learning studies in Alzheimer’s and related diseases is detecting and predicting neurodegeneration to provide early detection and accurate prognosis to support treatment and intervention. The main interests of this interdisciplinary field can be roughly categorized into three areas:
- Classification of various stages of AD. This area targets diagnosis or efficient progression monitoring. Current studies mostly focus on AD, MCI subtypes, and normal cognitive controls (NC). A few studies contain the subjective cognitive decline (SCD) stage before MCI.
- Predicting MCI conversion. This area is mainly approached by formulating prediction as a classification problem, which usually involves defining MCI converters and non-converters based on a time threshold from the initial diagnosis. Some studies also aim at the prediction of time-to-conversion for MCI to AD.
- Prediction of clinical measures. This area aims at producing surrogate biomarkers to reduce cost or invasivity, e.g., neuroimaging to replace lumbar puncture. Prediction of clinical measures, e.g., ADAS-Cog13 [72] and ventricular volume [73], is also used for longitudinal studies and attempts to achieve a more comprehensive evaluation of disease progression and model performance benchmarking.
-
Classification of various stages of AD. This area targets diagnosis or efficient progression monitoring. Current studies mostly focus on AD, MCI subtypes, and normal cognitive controls (NC). A few studies contain the subjective cognitive decline (SCD) stage before MCI.
-
Predicting MCI conversion. This area is mainly approached by formulating prediction as a classification problem, which usually involves defining MCI converters and non-converters based on a time threshold from the initial diagnosis. Some studies also aim at the prediction of time-to-conversion for MCI to AD.
-
Prediction of clinical measures. This area aims at producing surrogate biomarkers to reduce cost or invasivity, e.g., neuroimaging to replace lumbar puncture. Prediction of clinical measures, e.g., ADAS-Cog13 [75] and ventricular volume [76], is also used for longitudinal studies and attempts to achieve a more comprehensive evaluation of disease progression and model performance benchmarking.
There are also other areas of interest, including knowledge discovery, where studies attempt to understand AD through data [74][77]. Another area of interest is phenotyping and sample enrichment for clinical trials of treatments [75][78], where DL models are used to select patients that will likely respond to treatment and prevent ineffective or unnecessary treatment [76][79]. Interest also lies in segmentation and preprocessing, where DL models are applied to achieve higher performance or efficiency than conventional pipelines [77][80].
