Oral delivery of tissue-specific autoantigens may be helpful for the clinical prevention of spontaneous autoimmune diabetes. However, the therapeutic potential has been restricted by the need for recurrent delivery of large amounts of autoantigen, and tolerance is often less successful in already sensitized hosts. These limitations were overcome by transporting chemically conjugated autoantigens for the induction of oral tolerance utilizing the nontoxic B subunit of cholera toxin (CTB).
- CTB
- Adjuvant
- Plant-derived Vaccines
- IDO1
1. Introduction
2. CTB as an Adjuvant
The gram-negative bacterium Vibrio cholerae secretes a soluble toxin known as cholera toxin. Severe cholera, a potentially fatal diarrheal reaction, can be brought on by V. cholerae (especially O1 serogroups). Parts of the cholera toxin may have therapeutic characteristics, even though V. cholerae infection can be fatal. The two proteins that make up the cholera toxin are subunit A (CTA), which is present in the complex as a monomer, and subunit B (CTB), which forms a pentamer. The cholera toxin was first recognized as a dual protein by Lönnroth and Holmgren [13]. Following those of the cholera toxin and CTB, structural investigations of the closely related heat-labile enterotoxin from Escherichia coli were used to support these preliminary findings. Using five CTB monomers, a ring-like structure is created. Each monomer interacts with two neighboring molecules via salt bridges and hydrogen bonds without being covalently bonded to one another. In the middle of this pentameric arrangement, there are five alpha helices, one from each monomer, creating the wall of a tunnel-like structure. With a noticeable C-terminal alpha helical extension, CTA has a globular shape. When this extension is put into the tunnel made by the CTB pentamer, which only accepts one CTA molecule, the cholera toxin’s final AB5 hexameric structure is formed [14]. CTA is originally synthesized as a single protein chain, but a proteolytic cleavage splits it into two subunits, CTA1 and CTA2, which remain together in the hexamer until the complex enters the host cells and reaches the endoplasmic reticulum. CTA1 represents the globular component of CTA, and CTA2 is the projecting alpha helix that the pentamer inserts into the tunnel. Cholera toxin is recognized by its receptor, monosialotetrahexosylganglioside (GM1), which controls cellular absorption [15]. The identity of GM1 as a receptor for cholera toxin was described by Holmgren and colleagues in 1973 [13]. Each CTB monomer typically interacts with one pentasaccharide, even though each pentasaccharide extends its contact to an adjacent CTB molecule. This explains why CTB needs to take on a pentameric configuration in order to perform at its best. Only after numerous GM1 gangliosides have been simultaneously contacted does the improved avidity combine with the optimal sugar binding of two B subunits. The ability of the pentamer’s B subunits to engage with one another is enhanced by the pentasaccharide-CTB interaction [14][16][17]. Given that CTB is non-toxic and that its receptor is distributed throughout cells, this chemical makes an excellent delivery system. Numerous different cell types, including neurons, have GM1 [18]. When CTB is utilized in oral vaccination formulations, the presence of this ganglioside on antigen-presenting cells and the gut epithelial cells, which function as an important route of CTB absorption, is relevant. Apart from macrophages and dendritic cells [19], GM1 is also expressed by B cells, and upon CTB binding, expression of MHC class II is increased [20]. This dramatically increases the organism’s exposure to compounds related to and connected with CTB antigens. There are normally very few Ag-specific B cells in the entire B cell repertoire that are capable of efficiently capturing Ag through the B cell receptor (BCR), detecting the Ag, and directing it towards the MHC class II loading compartment in a homeostatic condition. Ag uptake by CTB and GM1 avoids this gap and may turn macrophages, dendritic cells, and the whole B cell compartment into potential antigen presenters. Silkworms have been used to successfully produce more CTB fusion proteins. In one study, CTB fused to the 42 amino acid version of the amyloid-peptide A42 in this system was utilized to successfully diminish A42 deposition in the brain of animals used to imitate Alzheimer’s disease [21], and when given orally to the nonobese diabetic (NOD) mouse, a relevant type 1 diabetes model, a fusion product of CTB and insulin in the same system has been asserted to decrease insulitis and the formation of diabetes [22][23][24]. CTB is utilized as an adjuvant, and in order to do so successfully, it must be recombinantly fused to the target antigen. The C-terminal fusion to CTB was compared to the N-terminal fusion in a systemic analysis [25]. In this investigation, protein G from Streptococcus, a 25 KD fusion partner, had its serum albumin binding region (BB) fused either N- or C-terminally, or both at once. All three proteins were produced and formed pentamers, but the C-terminal fusion protein formed the best pentamers and had the highest affinity for GM1. This indicates that CTB is a very tolerant fusion partner. In the case that either CTB does not fold correctly, the pentamer does not form, or the ability to bind to GM1 is compromised, if this construct is co-expressed with wild-type CTB, an alternate approach can be utilized by substituting CTA1 with Ag and attaching it N-terminally to CTA2 [26]. The formation of a CTB pentamer allows CTA2 to enter through the tunnel created by CTB, leaving Ag sticking out of the ring structure. In light of the fact that this build keeps the CTB moiety unaltered, the GM1 binding by the pentamer is ideal. This technique also decreases the molecular ratio of Ag to the CTB pentamer from 5:1 to 1:1, which must be taken into account when comparing vaccine dosages of various fusion proteins. If numerous Ag molecules or peptide epitopes are not fused to one another along with CTA2, this method also results in a reduction of the molecular ratio of Ag to the CTB pentamer. To prevent recombinant fusion, CTB and Ag can either be chemically coupled or supplied concurrently without being physically joined (Table 1). The protein can also be expressed and purified, or if expressed in plants, the purification phase can be omitted by developing an edible plant vaccine. Adding CTB to DNA vaccines is another way to prevent protein purification [27].| Antigen. | Fused to CTB | Animal Model, Pathogen or Route | Response | Reference |
|---|---|---|---|---|
| Human Proinsulin | C-terminal | Monocyte-derived Dendritic cells (moDCs) Human, in vitro | Upregulation of IDO1, Tolerogenic effect on DCs | [28][29] |
| Human Glutamic Acid Decarboxylase 55 (GAD55) | C-terminal, Recombinant vaccinia virus | NOD mouse, i.p., +/− CFA | Comparing the same vaccine without CFA to the same vaccine, diabetes was reduced by 50–20%. | [30][31][32] |
| Insulin | C-terminal, +/− GFP | NOD Mouse | 50 % reduction in T1D incidence | [33] |
| Myelin Basic Protein (MBP) | C-terminal | 3X TgAD (Alzheimer’s Disease) Mice, Oral | 70% less amyloid buildup in the hippocampus and cerebral cortex | [34] |
3. CTB in Transgenic Plants
Transgenic plants that express foreign proteins for industrial or therapeutic use can serve as an economical replacement for production techniques based on fermentation. Specific vaccines have been developed as a result of the transient or persistent expression of foreign genes in plants. Recent studies have shown that bacterial and viral pathogen antigens can be produced in plants while maintaining their original immunogenic characteristics. Transgenic potato tubers that produced a bacterial antigen caused humoral and mucosal immune reactions when provided as food. These results provide “proof of concept” for the synthesis of vaccines from plants [1][35][36][37]. Recombinant cell culture expression systems can be used to make subunit vaccines; however, these systems require fermentation technology and strict purification techniques to produce enough recombinant protein for oral administration. The cost of producing innovative oral vaccinations in underdeveloped countries may be prohibitive even with technological breakthroughs. An “edible vaccine,” created by transgenic plants that express antigens in their edible tissue, might be consumed to provide immunity. In contrast to the bacterially expressed form, CTB has been successfully expressed in a variety of plant systems, including tobacco (Nicotiana benthamiana), where the protein obtains glycosylated, most likely at position Asn25, which has no effect on the pentamer formation [38]. In this case, CTB was fused to the membrane proximal (ectodomain) region of gp41 (MPR649–684) and constituted about 0.14 percent of the total soluble protein, or around 20 mg per kg of fresh leaf material. The protein was immunogenic, and the authors noted that three doses were sufficient to raise vaginal IgA toward the protein, something they were unable to do in prior studies using bacterially expressed protein. Despite the lack of a direct side-by-side comparison with the bacterial version of the same construct in the study [38][39][40]. Expression of CTB has also been successful in rice to serve as a cholera vaccine [41][42][43], and when taken orally, it proved protective in mice and macaques. Instead of achieving its intended outcome of reducing the expression of rice allergens that might have negative effects on subjects, RNAi increased CTB expression in these plants by a factor of three. Concurrently with this, CTB’s intracellular localization changed. Plants with RNAi suppression were shown to have CTB in the cell wall, plasma membrane, and cytoplasm [44]. A different approach to subcellular localization in Nicotiana, i.e., expression in chloroplasts, has been chosen by Lakshmi et al. [45]. This was done by cloning CTB fused to Mycobacterium tuberculosis epitopes, specifically the 6 KD early secretory antigenic target (ESAT-6) and Mtb72F (a protein formed by the fusion of two tuberculosis antigens, Mtb32 and Mtb39), downstream of the psbA promoter that normally regulates expression of the protein D1 of Photosystem II. However, in that study, it was not examined whether the vaccine induced a protective immune response or not. Functional GM1-binding pentamers were created by CTB in chloroplasts. It has also been successful to achieve CTB expression in the chloroplast through the use of targeting vectors that led to the insertion of CTB-coding DNA into chloroplast DNA. The expressed protein was found to make up 4% of the whole protein in the plant, and functional pentamers accumulated in the chloroplasts. However, as an entire plant extract rather than purified CTB was employed, no thorough comparative findings on the GM1 binding capacity could be formed. Both the bacterially expressed CTB and plant-expressed pentamers bound to GM1 in an ELISA format [37][45]. Transgenic potatoes were modified to produce cholera toxin B subunit (CTB) pentamers with affinity for GM1-ganglioside. Oral vaccination-treated mice produced intestinal and serum antibodies against CTB. Mucosal antibody titers rapidly declined after the previous immunization, but they gradually increased again following an oral transgenic potato booster. Cholera holotoxin (CT) had a cytotoxic effect on Vero cells until serum from animals immunized with transgenic potato tissues neutralized it. After intraileal injection with CT, the mice that had been vaccinated with plants showed a reduction of up to 60% in the buildup of diarrheal fluid in the small intestine. Protection against CT was based on blocking enterotoxin’s capacity to bind to the cell-surface receptor GM1-ganglioside [1][35]. Agrobacterium tumefaciens-mediated transformation was used to create transgenic potato plants using the expression vector pPCV70lluxF containing either the human insulin cDNA (INS) or the CTB-INS fusion gene, as described by Arakawa et al. [35]. The bidirectional mannopine synthase P2 promoter was placed next to a plant expression vector that contained a bacterial luciferase AB fusion gene (luxF) connected to the P1 promoter. The cholera toxin B subunit protein (CTB), which is coupled to an ER retention signal (SEKDEL), is encoded by this gene. Potato leaf explants were transformed by the vector-carrying Agrobacterium tumefaciens, and kanamycin-resistant plants were subsequently produced. The genomic DNA of bioluminescent plants’ CTB-SEKDEL fusion gene was located using polymerase chain reaction amplification. An immunoblot investigation revealed that oligomeric CTB molecules were the most frequently extracted molecular species from transgenic potato leaf and tuber tissues and that the CTB protein generated from plants was antigenically identical to the CTB protein found in bacteria. As with CTB created by bacteria, plant-made CTB splits into monomers when exposed to heat or acid. The highest level of CTB protein was discovered in auxin-induced transgenic potato leaf and tuber tissues, corresponding to about 0.3 percent of the total soluble plant protein. Enzyme-linked immunosorbent assay methods used in that study showed that plant-produced CTB protein had a specific affinity for GM1-ganglioside, the cholera toxin’s natural membrane receptor [1][16][17][35][40][46]. In potato tissues, CTB protein accumulates and forms an oligomeric form that retains its original biochemical and immunological properties when the SEKDEL signal is present.-
How the fusion protein of cholera toxin B subunit and insulin, made from potatoes, prevents the onset of autoimmune diabetes.
It has been demonstrated that oral administration of disease-specific autoantigens can halt or postpone the development of autoimmune disease symptoms[11, 12, 23, 43, 48, 49]. Arakawa et al. have generated transgenic potato plants synthesize human insulin, which is a major insulin-dependent diabetes mellitus autoantigen, at levels up to 0.05% of total soluble protein[2, 3, 29]. In this study, insulin was coupled to the C-terminus of the cholera toxin B subunit (CTB) to facilitate the distribution of plant-produced insulin to the lymphoid tissues associated with the gut. Transgenic potato tubers produced pentameric CTB-insulin fusion, which conserved the native antigenicity of both CTB and insulin, as well as 0.1 percent of the total soluble protein. Nonobese diabetic mice given CTB-insulin fusion protein-infused altered potato tuber tissues showed markedly reduced pancreatic islet inflammation (insulitis) and a postponed onset of clinical diabetes. When insulin- or CTB-producing transgenic potato tissues were fed on their own, neither insulitis nor diabetic symptoms were noticeably lessened. The results of the experiments suggest that food plants can be used as both a source and a vehicle for immune-tolerization against this T cell-mediated autoimmune illness.
The pentameric shape of the CTB fusion protein increases the molar concentration of the antigen per CTB pentamer molecule in addition to enabling site-specific delivery and presentation of conjugated proteins to the GALT. The traditional necessity for high levels of antigen biosynthesis in the food plant for effective vaccine manufacture may be greatly decreased as a result of the enhanced antigen concentration in the GALT. The proinsulin was coupled with CTB via the flexible hinge peptide to minimize steric hindrance between the CTB and insulin moieties and enhance CTB subunit production in plant cells[50].
On the basis of the NOD mice's induction of both systemic and intestinal anti-CTB antibodies after being fed altered potato tubers that contain the CTB-INS fusion protein, it is possible that the fusion protein was successfully transported to the intestine immune system. The discovery of an elevated serum anti-insulin IgG antibody titer provided more evidence in favor of this assertion. The humoral reaction mounted against the CTB and insulin proteins may be a secondary outcome of oral tolerance's stimulation of Th cells [12, 39, 43, 51, 52].
Serum anti-insulin IgG1 antibodies were more frequently induced when mice were fed transgenic potato tissues that produced the CTB-INS fusion protein than IgG2a antibodies, demonstrating that the immune response was biased toward an insulin-specific Th2 lymphocyte response and that the observed oral tolerance was mediated by active suppression [2, 53].
According to this study, autoimmune diabetes can be successfully prevented by giving NOD mice very small amounts of food that contains insulin made by plants and conjugated with a CTB subunit. The significance of oral antigen dose and the contribution of CTB to the induction of oral tolerance are highlighted by the efficacy of the plant-produced CTB-INS fusion protein at doses at least 100 times lower than those generally reported for unconjugated autoantigens[48, 54].
Due to their ability to control tolerance to food antigens, limit reactivity to the gut microbiota, and mediate tolerance to food antigens, dendritic cells (DCs) are essential for an efficient response to intestinal infections. Intestinal DCs can promote regulatory and effector T cell responses despite their diversity. The actions taken by functionally different DC subsets and how environmental cues influence those actions determine the results in a particular circumstance. DCs continuously sample luminal content to look for infections, but the relevance of the various paths by which this occurs is not entirely known. The distinct traits of intestinal DCs are controlled by local host, nutritional, and microbial cues. The ability to produce all-trans retinoic acid (RA) and activate T lymphocytes with gut tropism are two examples of these traits[55]. Due to their ability to activate TGF beta and generate RA, intestinal DC subsets are potent inducible Treg inducers in the steady state. Responses elicited by steady-state intestinal DCs are not entirely regulatory in character, despite the presence of effector T cells with a preference for commensal bacteria in healthy mucosa and their ability to be locally controlled to stop inflammation[56].
To enhance effector responses in an infection or maintain inflammation in a disease, intestinal DCs probably need to both change the current DC population and attract new populations. The etiology of inflammatory bowel disease can be linked to immune pathways, and in inflamed intestinal tissue, DCs show enhanced microbial recognition machinery expression, activation, and mediator synthesis. Intestinal DCs may be targeted for better vaccination responses or disease treatment[57].
Tryptophan is an essential amino acid, and indoleamine 2, 3-dioxygenase (IDO1) is the first and only catabolic enzyme that can degrade it. IDO breaks down the aromatic indole ring of tryptophan, resulting in the production of a number of tryptophan breakdown metabolites known as "kynurenines." These chemicals have been found to significantly influence immune regulation. Since tryptophan must be received through diet, managing how it is metabolized can significantly affect how the body uses energy and how the immune system responds[58]. By depleting tryptophan, reducing cellular energy generally, and producing released kynurenines, which are known to effectively trigger pro-inflammatory T-cell death, indoleamine 2, 3-dioxygenase was discovered to limit DC maturation(Figure 1)[41].
Th17 cells are characterized by their production of interleukin-17 (IL-17) and other pro-inflammatory cytokines. They are involved in immune responses against pathogens at mucosal surfaces, but their dysregulation can lead to chronic inflammation and tissue damage, promoting autoimmune disorders[59].
According to earlier research, transgenic DCs with high levels of IDO1 expression and tryptophan metabolites (such as l-kynurenine, 3-hydroxykynurenine, and 3-hydroxyanthranilic acid) can permanently decrease allogeneic T-cell proliferation in vitro including Th 17[60-62] and lead to an increased recruitment of CD4+CD25+Foxp3+ T regulatory (Treg) cells within the site of antigen presentation[63-65]. IDO1 was found to be significantly up-regulated in vaccine-inoculated DCs in a recent study using immature human dendritic cells (iDC) co-cultured with CTB-INS. The immunosuppressive enzyme's production was greatly boosted by the CTB-INS fusion protein when compared to DCs that had been exposed to proinsulin, CTB, or an unlinked mixture of the two proteins[41].
According to current research, CTB-INS promotes TNFR signaling and induces IDO1 production in human DCs, which may be the mechanism by which it modulates DC activation. Examining IDO1 production caused by CTB-INS in vaccine-stimulated DCs was made possible by blocking the TNFR pathway[42]. In this recent study, peptides comprising the amino acid sequences of the TRAF2, 3 and TRAF6 binding sites for CD40 were treated with monocyte derived DCs. Following that, CD40 ligand (CD154) and CTB-INS were used to activate the DCs. Blocking peptides for CD40-TRAF2, 3, and CD40-TRAF6 were added, and this prevented IDO1 production from being upregulated in response to either CD154 ligand or CTB-INS. Combining TRAF2, 3 and TRAF6 inhibitors resulted in the highest decrease in IDO1 production.
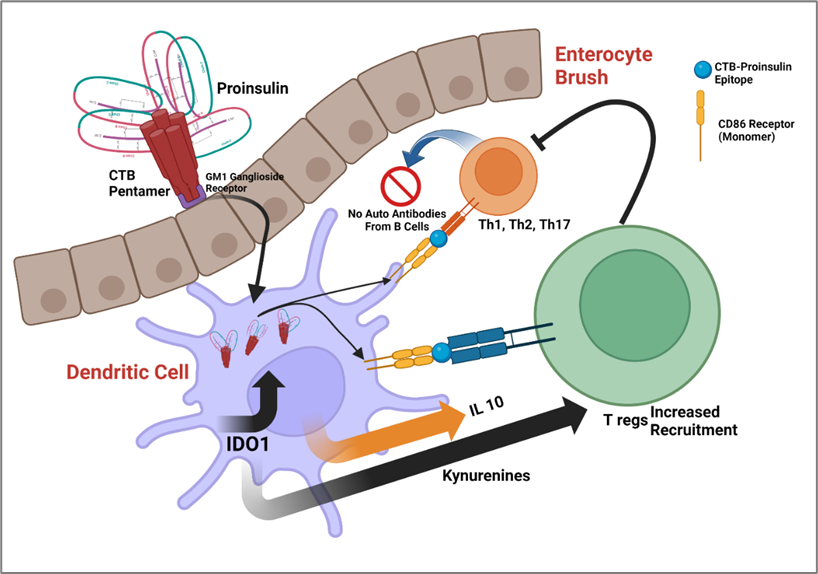
Figure 1. Proposed Mechanism for CTB-INS edible vaccine. Created with BioRender.com. Oral Administration: The CTB-INS vaccine is orally administered, typically through ingestion or ingestion of a plant-based product containing the vaccine. Uptake in the Gut: After oral administration, the vaccine components, including the cholera toxin B subunit (CTB) and the insulin antigen (INS), are taken up by specialized cells in the gut, such as M cells or dendritic cells (DCs). Presentation to Immune Cells: The gut-associated DCs capture the vaccine antigens and migrate to the lymphoid tissues, such as Peyer's patches, mesenteric lymph nodes, or other lymphoid organs. Production of IDO: Indoleamine 2,3-dioxygenase (IDO) is an enzyme involved in the metabolism of tryptophan. The presence of IDO and IL10 induced by the CTB-INS vaccine promotes the migration of Tregs in the gut. IDO plays a key role in immune tolerance by regulating T cell responses and maintaining immune balance through Treg migration, which play a crucial role in immune regulation and tolerance via the inhibition of Th1, Th2 and Th17 and B cell autoantibody production.
The specific immune-unresponsive condition is enhanced in mice after oral administration of the cholera toxin B (CTB) subunit conjugated to the autoantigen insulin. Oral tolerance is what it is known as, and it can suppress autoimmune type 1 diabetes (T1D). The mechanism through which the CTB-insulin (CTB-INS) protein functions as a treatment for T1D in vivo, however, is still unknown. However, recent articles have provided a number of hints. Within organized lymphoid tissue, intestinal cDCs are involved in mediating tolerance to dietary antigens, limiting responsiveness to the gut microbiota, and being necessary for an effective response to intestinal infections. Although intestinal DCs are diverse, they together induce responses from regulatory and effector T cells. In nonobese diabetic (NOD) mice, oral treatment of the CTB-INS protein was recently found to generate exceptional tolerance, postpone the onset of diabetic symptoms, and decrease the start of T1D. Through the non-canonical NF-ĸB pathway, it has been demonstrated to increase levels of the immunosuppressive and tolerogenic enzyme IDO1 in dendritic cells and reduce levels of CD86 costimulatory factors[41, 42]. The by-products of resulting tryptophan catabolism known as kynurenines are known to cause the increase of CD4+CD25+Foxp3+ T regulatory (Treg) cells within the GALT.
-
Conclusion
Edible vaccines are ingested as food and offer advantages in safety and production. They rely on the plant's life cycle and hold potential for controlling diseases. The mechanism of action is still being studied, but evidence suggests that oral administration can induce immune responses and suppress autoimmune diabetes. Intestinal dendritic cells play a role in tolerance and response to infections. While concerns exist about genetically modified plants and cross-contamination, thorough monitoring can address these risks. Edible vaccines have significant benefits and require further research for enhanced disease control. Plant-based vaccine production holds promise for preventing global epidemics.
References
- Arakawa, T.; Chong, D.K.; Langridge, W.H. Efficacy of a food plant-based oral cholera toxin B subunit vaccine. Nat. Biotechnol. 1998, 16, 292–297.
- Arakawa, T.; Yu, J.; Langridge, W.H. Food plant-delivered cholera toxin B subunit for vaccination and immunotolerization. Adv. Exp. Med. Biol. 1999, 464, 161–178.
- Yuki, Y.; Nojima, M.; Hosono, O.; Tanaka, H.; Kimura, Y.; Satoh, T.; Imoto, S.; Uematsu, S.; Kurokawa, S.; Kashima, K.; et al. Oral MucoRice-CTB vaccine for safety and microbiota-dependent immunogenicity in humans: A phase 1 randomised trial. Lancet Microbe 2021, 2, e429–e440.
- Trentham, D.E.; Dynesius-Trentham, R.A.; Orav, E.J.; Combitchi, D.; Lorenzo, C.; Sewell, K.L.; Hafler, D.A.; Weiner, H.L. Effects of oral administration of type II collagen on rheumatoid arthritis. Science 1993, 261, 1727–1730.
- Weiner, H.L.; Mackin, G.A.; Matsui, M.; Orav, E.J.; Khoury, S.J.; Dawson, D.M.; Hafler, D.A. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science 1993, 259, 1321–1324.
- Weiner, H.L.; Friedman, A.; Miller, A.; Khoury, S.J.; al-Sabbagh, A.; Santos, L.; Sayegh, M.; Nussenblatt, R.B.; Trentham, D.E.; Hafler, D.A. Oral tolerance: Immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu. Rev. Immunol. 1994, 12, 809–837.
- Zhang, Z.J.; Davidson, L.; Eisenbarth, G.; Weiner, H.L. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc. Natl. Acad. Sci. USA 1991, 88, 10252–10256.
- Carel, J.C.; Bougnères, P.; Vardi, P. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. J. Endocrinol. Investig. 1994, 17, 573–580.
- Sun, J.B.; Holmgren, J.; Czerkinsky, C. Cholera toxin B subunit: An efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc. Natl. Acad. Sci. USA 1994, 91, 10795–10799.
- Holmgren, J.; Adamsson, J.; Anjuère, F.; Clemens, J.; Czerkinsky, C.; Eriksson, K.; Flach, C.F.; George-Chandy, A.; Harandi, A.M.; Lebens, M.; et al. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol. Lett. 2005, 97, 181–188.
- Sun, J.B.; Czerkinsky, C.; Holmgren, J. Mucosally induced immunological tolerance, regulatory T cells and the adjuvant effect by cholera toxin B subunit. Scand. J. Immunol. 2010, 71, 1–11.
- Sun, J.B.; Rask, C.; Olsson, T.; Holmgren, J.; Czerkinsky, C. Treatment of experimental autoimmune encephalomyelitis by feeding myelin basic protein conjugated to cholera toxin B subunit. Proc. Natl. Acad. Sci. USA 1996, 93, 7196–7201.
- Lönnroth, I.; Holmgren, J. Subunit structure of cholera toxin. J. Gen. Microbiol. 1973, 76, 417–427.
- Sixma, T.K.; Kalk, K.H.; van Zanten, B.A.; Dauter, Z.; Kingma, J.; Witholt, B.; Hol, W.G. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J. Mol. Biol. 1993, 230, 890–918.
- Chester, M.A. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids–recommendations 1997. Eur. J. Biochem. 1998, 257, 293–298.
- Schön, A.; Freire, E. Thermodynamics of intersubunit interactions in cholera toxin upon binding to the oligosaccharide portion of its cell surface receptor, ganglioside GM1. Biochemistry 1989, 28, 5019–5024.
- Goins, B.; Freire, E. Thermal stability and intersubunit interactions of cholera toxin in solution and in association with its cell-surface receptor ganglioside GM1. Biochemistry 1988, 27, 2046–2052.
- Kozireski-Chuback, D.; Wu, G.; Ledeen, R.W. Developmental appearance of nuclear GM1 in neurons of the central and peripheral nervous systems. Brain Res. Dev. Brain Res. 1999, 115, 201–208.
- Moreno-Altamirano, M.M.; Aguilar-Carmona, I.; Sánchez-García, F.J. Expression of GM1, a marker of lipid rafts, defines two subsets of human monocytes with differential endocytic capacity and lipopolysaccharide responsiveness. Immunology 2007, 120, 536–543.
- Francis, M.L.; Ryan, J.; Jobling, M.G.; Holmes, R.K.; Moss, J.; Mond, J.J. Cyclic AMP-independent effects of cholera toxin on B cell activation. II. Binding of ganglioside GM1 induces B cell activation. J. Immunol. 1992, 148, 1999–2005.
- Li, S.; Wei, Z.; Chen, J.; Chen, Y.; Lv, Z.; Yu, W.; Meng, Q.; Jin, Y. Oral administration of a fusion protein between the cholera toxin B subunit and the 42-amino acid isoform of amyloid-β peptide produced in silkworm pupae protects against Alzheimer's disease in mice. PLoS ONE 2014, 9, e113585.
- Gong, Z.; Jin, Y.; Zhang, Y. Suppression of diabetes in non-obese diabetic (NOD) mice by oral administration of a cholera toxin B subunit-insulin B chain fusion protein vaccine produced in silkworm. Vaccine 2007, 25, 1444–1451.
- Gong, Z.H.; Jin, H.Q.; Jin, Y.F.; Zhang, Y.Z. Expression of cholera toxin B subunit and assembly as functional oligomers in silkworm. J. Biochem. Mol. Biol. 2005, 38, 717–724.
- Gong, Z.; Jin, Y.; Zhang, Y. Oral administration of a cholera toxin B subunit-insulin fusion protein produced in silkworm protects against autoimmune diabetes. J. Biotechnol. 2005, 119, 93–105.
- Liljeqvist, S.; Ståhl, S.; Andréoni, C.; Binz, H.; Uhlén, M.; Murby, M. Fusions to the cholera toxin B subunit: Influence on pentamerization and GM1 binding. J. Immunol. Methods 1997, 210, 125–135.
- Hajishengallis, G.; Hollingshead, S.K.; Koga, T.; Russell, M.W. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immunol. 1995, 154, 4322–4332.
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines-fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250.
- Mbongue, J.C.; Nicholas, D.A.; Zhang, K.; Kim, N.S.; Hamilton, B.N.; Larios, M.; Zhang, G.; Umezawa, K.; Firek, A.F.; Langridge, W.H. Induction of indoleamine 2, 3-dioxygenase in human dendritic cells by a cholera toxin B subunit-proinsulin vaccine. PLoS ONE 2015, 10, e0118562.
- Kim, N.S.; Mbongue, J.C.; Nicholas, D.A.; Esebanmen, G.E.; Unternaehrer, J.J.; Firek, A.F.; Langridge, W.H. Chimeric Vaccine Stimulation of Human Dendritic Cell Indoleamine 2, 3-Dioxygenase Occurs via the Non-Canonical NF-κB Pathway. PLoS ONE 2016, 11, e0147509.
- Dénes, B.; Fodor, I.; Langridge, W.H. Autoantigens plus interleukin-10 suppress diabetes autoimmunity. Diabetes Technol. Ther. 2010, 12, 649–661.
- Langridge, W.; Dénes, B.; Fodor, I. Cholera toxin B subunit modulation of mucosal vaccines for infectious and autoimmune diseases. Curr. Opin. Investig. Drugs 2010, 11, 919–928.
- Dénes, B.; Fodor, I.; Langridge, W.H. Persistent suppression of type 1 diabetes by a multicomponent vaccine containing a cholera toxin B subunit-autoantigen fusion protein and complete Freund’s adjuvant. Clin. Dev. Immunol. 2013, 2013, 578786.
- Meng, Q.; Wang, W.; Shi, X.; Jin, Y.; Zhang, Y. Protection against autoimmune diabetes by silkworm-produced GFP-tagged CTB-insulin fusion protein. Clin. Dev. Immunol. 2011, 2011, 831704.
- Kohli, N.; Westerveld, D.R.; Ayache, A.C.; Verma, A.; Shil, P.; Prasad, T.; Zhu, P.; Chan, S.L.; Li, Q.; Daniell, H. Oral delivery of bioencapsulated proteins across blood-brain and blood-retinal barriers. Mol. Ther. 2014, 22, 535–546.
- Arakawa, T.; Chong, D.K.; Merritt, J.L.; Langridge, W.H. Expression of cholera toxin B subunit oligomers in transgenic potato plants. Transgenic Res. 1997, 6, 403–413.
- Mor, T.S.; Gómez-Lim, M.A.; Palmer, K.E. Perspective: Edible vaccines—A concept coming of age. Trends Microbiol. 1998, 6, 449–453.
- Daniell, H.; Lee, S.B.; Panchal, T.; Wiebe, P.O. Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J. Mol. Biol. 2001, 311, 1001–1009.
- Matoba, N.; Kajiura, H.; Cherni, I.; Doran, J.D.; Bomsel, M.; Fujiyama, K.; Mor, T.S. Biochemical and immunological characterization of the plant-derived candidate human immunodeficiency virus type 1 mucosal vaccine CTB-MPR. Plant Biotechnol. J. 2009, 7, 129–145.
- Matoba, N.; Magérus, A.; Geyer, B.C.; Zhang, Y.; Muralidharan, M.; Alfsen, A.; Arntzen, C.J.; Bomsel, M.; Mor, T.S. A mucosally targeted subunit vaccine candidate eliciting HIV-1 transcytosis-blocking Abs. Proc. Natl. Acad. Sci. USA 2004, 101, 13584–13589.
- Matoba, N.; Griffin, T.A.; Mittman, M.; Doran, J.D.; Alfsen, A.; Montefiori, D.C.; Hanson, C.V.; Bomsel, M.; Mor, T.S. Transcytosis-blocking abs elicited by an oligomeric immunogen based on the membrane proximal region of HIV-1 gp41 target non-neutralizing epitopes. Curr. HIV Res. 2008, 6, 218–229.
- Nochi, T.; Takagi, H.; Yuki, Y.; Yang, L.; Masumura, T.; Mejima, M.; Nakanishi, U.; Matsumura, A.; Uozumi, A.; Hiroi, T.; et al. Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc. Natl. Acad. Sci. USA 2007, 104, 10986–10991.
- Nochi, T.; Yuki, Y.; Katakai, Y.; Shibata, H.; Tokuhara, D.; Mejima, M.; Kurokawa, S.; Takahashi, Y.; Nakanishi, U.; Ono, F.; et al. A rice-based oral cholera vaccine induces macaque-specific systemic neutralizing antibodies but does not influence pre-existing intestinal immunity. J. Immunol. 2009, 183, 6538–6544.
- Yuki, Y.; Tokuhara, D.; Nochi, T.; Yasuda, H.; Mejima, M.; Kurokawa, S.; Takahashi, Y.; Kataoka, N.; Nakanishi, U.; Hagiwara, Y.; et al. Oral MucoRice expressing double-mutant cholera toxin A and B subunits induces toxin-specific neutralising immunity. Vaccine 2009, 27, 5982–5988.
- Kurokawa, S.; Kuroda, M.; Mejima, M.; Nakamura, R.; Takahashi, Y.; Sagara, H.; Takeyama, N.; Satoh, S.; Kiyono, H.; Teshima, R.; et al. RNAi-mediated suppression of endogenous storage proteins leads to a change in localization of overexpressed cholera toxin B-subunit and the allergen protein RAG2 in rice seeds. Plant Cell Rep. 2014, 33, 75–87.
- Lakshmi, P.S.; Verma, D.; Yang, X.; Lloyd, B.; Daniell, H. Low cost tuberculosis vaccine antigens in capsules: Expression in chloroplasts, bio-encapsulation, stability and functional evaluation in vitro. PLoS ONE 2013, 8, e54708.
- Carter, J.E.; Yu, J.; Choi, N.W.; Hough, J.; Henderson, D.; He, D.; Langridge, W.H. Bacterial and plant enterotoxin B subunit-autoantigen fusion proteins suppress diabetes insulitis. Mol. Biotechnol. 2006, 32, 1–15.
