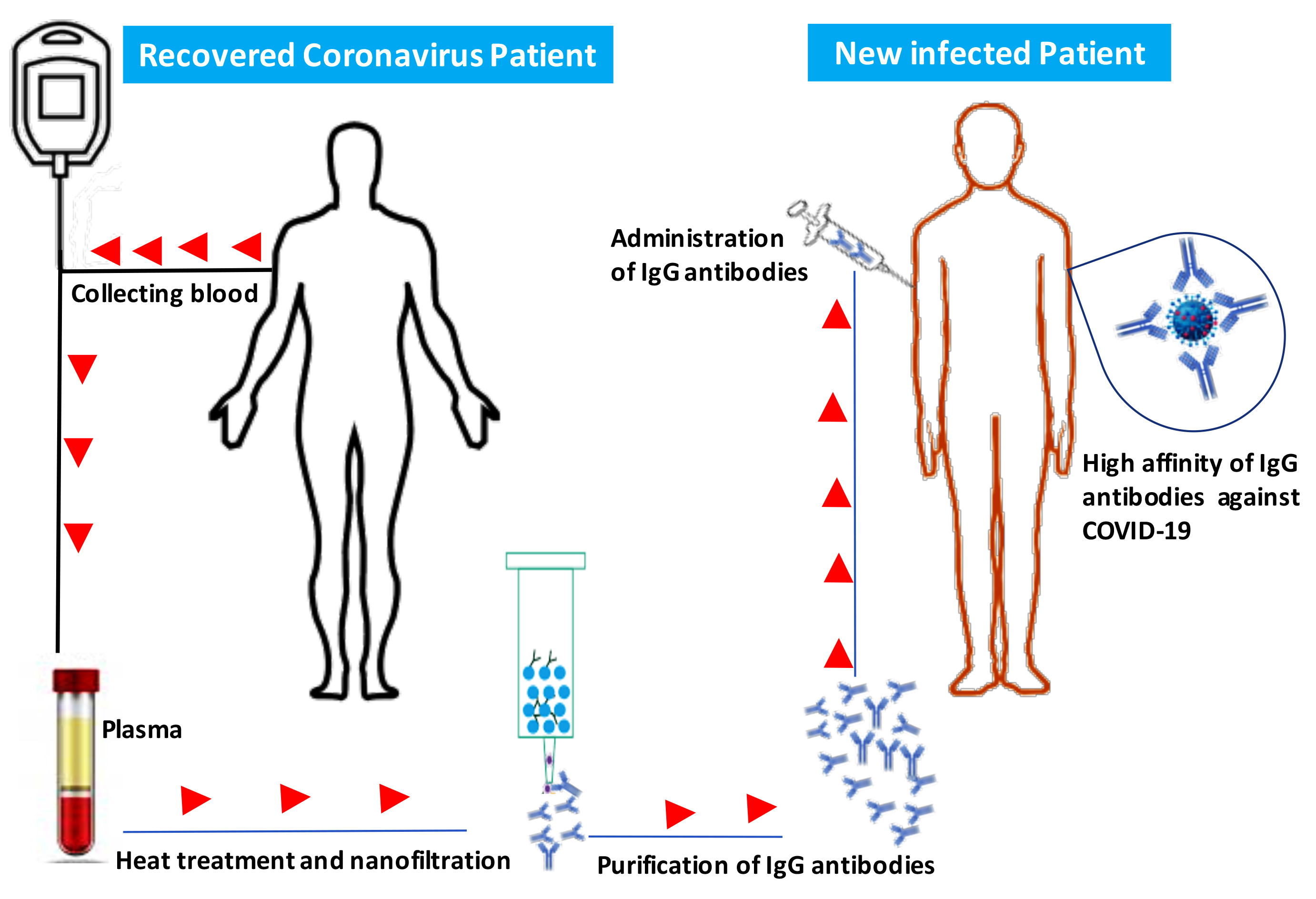
IThe emergG antibodies include two functional portions: the F(ab′)2 fragment, which is responsible for antigen recognition, and the crystallence of the novel coronavirus in Wuhan, China, which causes severe respiratory tract infections in humans (COVID-19), has become a global health concern. Most coronaviruses infect animals but can evolve into strains that cross the species barrier and infect humans. At the present, there is no single specific vaccine or efficient antiviral therapy against COVID-19. Recently, we showed that intravenous immunoglobulin (IVIg) treatment reduces inflammation of intestinal epithelial cells and eliminates overgrowth of the opportunistic human fungal pathogen Candida albicansin the murizne gut. Immunotherable fragment (Fc), which is important for activation ofpy with IVIg could be employed to neutralise COVID-19. However, the efficacy of IVIg would be better if the immune IgG antibodies were collected from the same city or the surrounding area of patients recovered from COVID-19 in order to increase the chance of neutralizing the virus. These immune IgG antibodies will be specific against COVID-19 by boosting the immune response by interacting with Fcγ receptors on B-cells and other innatin new infected patients. Different procedures may be used to remove or inactivate any possible pathogens from plasma of recovered Coronavirus patient-derived the immune cells. The Fc fragment also plays an important role in the activation of compleIgG, including solvent/detergent, 60°C heat-treatment, and nanofiltration. Overall, immunotherapy with immune IgG antibodies combined with antiviral drugs may be alternative treatment and in the clearance of microorganismsgainst COVID-19 until stronger options such as vaccines are available.
- COVID-19
- Coronavirus
- IgG
- IVIg
- Virus
- Immunotherapy
- SARS-CoV-2
1. Introduction
The emergence of the novel coronavirus in Wuhan, China, which causes severe respiratory tract infections in humans (COVID-19), has become a global health concern. Most coronaviruses infect animals but can evolve into strains that can also infect humans. Recently, we showed that intravenous immunoglobulin (IVIg) treatment reduces inflammation of intestinal epithelial cells and eliminates overgrowth of the opportunistic human fungal pathogen Candida albicans in the murine gut in association with downregulation of proinflammatory mediators combined with upregulation of anti-inflammatory cytokines [1].
Coronaviruses are enveloped positive-stranded RNA viruses belonging to the family Coronaviridae [2]. An envelope-anchored spike protein promotes coronavirus entry into host cells by first binding to a host receptor and then fusing viral and host membranes [2]. Whole-genome sequencing of viral RNA has revealed that the virus causing COVID-19 is phylogenetically related to the SARS-related coronaviruses first isolated in Chinese horseshoe bats during 2015‒2017 [3][4]. Researchers in Guangzhou, China, have recently suggested that pangolins are the probable animal source of the COVID-19 outbreak [5]. In terms of the interaction between the virus and its host, Lu et al. have reported that angiotensin-converting enzyme 2 (ACE 2) is most probably used by the spike protein of the SARS-CoV-2 as a receptor similar to that SARS-CoV [6].
Recently, Tang et al. showed that the SARS-CoV-2 has evolved into two major lineages—dubbed ‘L’ and ‘S’ types. The older ‘S-type’ appears to be milder and less infectious, while the ‘L-type’, which emerged later, spreads quickly and is currently more aggressive than the S-type [7]. Current symptoms reported for patients with COVID-19 have included mild to severe respiratory illness with fever, fatigue, cough, myalgia, and difficulty breathing [8]. Tyrrell et al. showed that infected respiratory epithelial cells by coronavirus become vacuolated and show damaged cilia that lead to production of inflammatory mediators, which increase nasal secretion and cause local inflammation and swelling [9]. These responses in turn stimulate sneezing, obstruct the airway, and raise the temperature of the mucosa [9].
Currently, there is no single specific vaccine or effective antiviral therapy against SARS-CoV-2. Several pharmaceutical and biotechnological companies are working on vaccine development and estimate that this vaccine will take years to develop and test before it can reach a large population. Additionally, there are currently no approved treatments for any coronavirus disease, including COVID-19. Several antiviral drugs are being tested, and initial findings are expected soon. Individuals with weakened immune systems appear to be at greater risk of developing complications associated with COVID-19. Immunotherapy using IgG in combination with antiviral drugs could be used to treat or prevent COVID-19 and to strengthen our immune response against this virus [10][11]. IgG antibodies include two functional portions: the F(ab′)
2
2. Description
IVIg is a pool of IgG from thousands of healthy donors, and exposure of individual donors to endemic infectious diseases, vaccines, and ubiquitous microorganisms participates in the production of IgG antibodies against different microorganisms and their products [13][14][15].
IVIg has been used to treat patients with autoimmune and chronic inflammatory diseases, such as dermatomyositis, Kawasaki disease, multiple sclerosis, lupus, chronic lymphocytic leukemia, and idiopathic thrombocytopenic purpura [16][17][18]. Furthermore, IVIg has also been used as an anti-infectious agent against viruses, bacteria, and fungi in human patients and experimental models [13][19][20][21]. IVIg treatment may result in some adverse events, which are associated with specific immunoglobulin preparations and individual differences, but many clinical and experimental studies show that switching from IVIg to subcutaneous immunoglobulin can minimize these adverse events [22][23][24].
IVIg plays an important role in the prevention of infectious episodes in primary immunodeficient patients, and the beneficial effects of these antibodies in the treatment of infectious diseases goes beyond simple neutralization of microorganisms or their toxins. Anti-inflammatory pathways are also critical for protection against infection [25].
IVIg may modulate the immune response via multiple mechanisms, including blocking a wide array of proinflammatory cytokines, Fc-gamma receptors (FcγRs), and leukocyte adhesion molecules, suppressing pathogenic Th1 and Th17 subsets, and neutralizing pathogenic autoantibodies [26][27][28]. IVIg can also expand regulatory T-cells by induction of cyclo-oxygenase-2-dependent prostaglandin E2 production in dendritic cells [29].