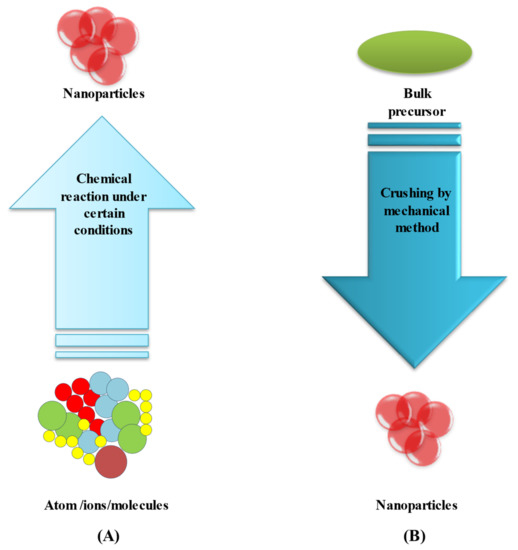
3.2. Top-Down Method
This method is used to transform bulk material into tiny nanoparticles. Top-down techniques are straightforward to employ. However, they are unsuccessful when producing irregularly shaped and very small particles. The main disadvantage of the top-down approach is the difficulty in acquiring a suitable particle size and shape
[66][67][95,96]. Laser ablation is the most controllable top-down approach. Bulk material is treated with a laser beam (in this case, a bimetallic Au–Ag alloy). Under optimum conditions, well-dispersed bimetallic Au–Ag NPs can be synthesized, which can then be fractionated and surface-functionalized. A two-step synthesis, which involves laser irradiating a combination of silver and gold nanoparticles, is another alternative
[68][97].
4. Antibacterial Properties of Bimetallic Au–Ag NPs
Controlling the invasion of new bacterial infections, their increasing proliferative powers, and antibacterial resistance, all of which have major public health implications, necessitates the use of extremely potent antimicrobial agents. Due to their synergistic effects, broad spectrum of physiochemical properties, and various mechanisms of action, bimetallic nanoparticles synthesized by combining two distinct metals have recently emerged as having a promising antibacterial efficiency exceeding those of their monometallic counterparts. Consequently, Au–Ag bimetallic nanoparticles are of great importance in imaging, biomedical devices, and nanomedicine
[69][107].
In a study, Ding et al., synthesized Au–Ag core–shell NPs via a chemical route and investigated their antimicrobial efficacy. In this study, they reported the aggregation of Au–Ag core–shell NPs onto the bacterial surface, which led to improved imaging because of the improved two-photon photoluminescence. These nanoparticles were found to have antibacterial action against
S. aureus while being less harmful to human dermal fibroblasts
[70][81]. On the other hand, Bankura et al., reported the use of dextran as a reducing agent for the synthesis of Au–Ag alloy NPs and investigated their antimicrobial efficacy. The antibacterial activity of a 0.1 mg/mL concentration of Ag-Au alloy NPs was found to be significant against bacteria (
B. subtilis,
B. cereus,
E. coli, and
P. aeruginosa) with zones of inhibition of 24, 21, 17, and 20 mm
[71][108]. In another report, the author followed a photosynthetic route to synthesize Au–Ag alloy nanoparticles for the first time. The bioreduction material in the study was essential oil from
Coleus aromaticus. Gram-negative
E. coli and Gram-positive
S. aureus were used to test the antibacterial efficacy of the photosynthesized Au–Ag alloy nanoparticles. An inhibitory zone of 28 mm for the alloy nanoparticles (synthesized with 150 µL essential oil) demonstrated their strong bactericidal activity against
E. coli. An in vitro antioxidant assay of the herbal-deduced nanoparticles also exhibited intense free radical (superoxide, hydroxyl, and nitric oxide radicals) scavenging activity
[72][109]. Similarly, Amina et al., prepared Au–Ag alloy nanoparticles by using a microwave-assisted technique that utilized an extract of
Asparagus racemosus root. In addition, the green-synthesized bimetallic alloy nanoparticles were tested against five different bacterial strains (
Bacillus subtilis (ATCC 6633),
Escherichia coli (ATCC 25922), Klebsiella pneumonia (Urine),
Pseudomonas aeruginosa (ATCC 27853), and
Staphylococcus aureus (ATCC 25923). It was reported that
P. aeruginosa and
S. aureus strains were the most susceptible (highest zone of inhibition) towards Au–Ag alloy nanoparticles versus single metal nanoparticles synthesized with plant extract
[73][110]. Additionally, Gopinath et al., synthesized green bimetallic (Au–Ag) nanoparticles by using
Gloriosa superba aqueous leaf extract. It was demonstrated that the developed nanoparticles had higher antibacterial as well as antibiofilm activities against Gram-positive and Gram-negative bacteria. The authors found a significant zone of inhibition at 6.33 ± 0.33 mm and 5.33 ± 0.33 mm for
B. subtilis and
E. coli, respectively
[74][111].
In another study, recently developed biosynthesized Au–Ag NPs without the incorporation of a surfactant or stabilizing agent. It was observed that when the pH of a solution with
E. coli and Au ions was raised, Au nanomaterials were formed. Core–shell Au–Ag nanostructures were generated in an ordered manner after Ag ions combined with the Au core. The spectroscopic and microscopic analyses confirmed the structural composition of the biosynthetic bimetallic Au–Ag nanoparticles
[75][121]. In a similar study, Liu et al., reported that their bimetallic NPs showed stronger application possibilities in the superfast colorimetric monitoring of H
2O
2, photothermal treatments, and antimicrobial therapy. Without using 3,3′,5,5′-tetramethylbenzidine or peroxidase, their bimetallic Au–Cu NPs were able to sense H
2O
2 quickly and calorimetrically
[76][122]. Furthermore, Au–Ag NPs could improve antibacterial activity without increasing cytotoxicity, ensuring that silver could be used in clinical settings
[69][107]. In recent studies, Kalwar et al., created Au–Ag-NP-decorated cellulose nanofibers. Cellulose acetate nanofibers were made by electrospinning, and alkaline hydrolysis was used to deacetylate them. The Au–Ag NPs were coated on the surface of cellulose nanofibers using a dipping process to create an excellent wound dressing material. Furthermore, their antibacterial activity against
E. coli and
S. aureus was tested, and the Au–Ag NPs/cellulose was found to be a good antimicrobial material
[77][123].
Villalobos-Noriega et al., synthesized bimetallic core–shell Au–Ag NPs by a green approach. Root extract of
Rumex hymenosepalus containing catechins and stilbenes acted as a reducing agent in the NPs synthesis. The growth kinetics of microorganisms was analyzed by the Gompertz model. The findings suggested that silver NPs and bimetallic Au–Ag NPs had a dose-dependent effect on the lag phase and growth rate of
E. coli and
Candida albicans, with the Au–Ag NPs having a better response
[78][79][120,124].
5. Bimetallic Nanoparticles Targeting Multidrug-Resistant Bacteria
Multidrug-resistant (MDR) bacteria are widely recognized as one of the most serious current public health issues, killing an estimated 700,000 people each year throughout the world
[80][125]. Furthermore, treating MDR bacteria with ineffective antibiotics promotes the expansion of bacterial tolerance. For example, almost more than 50% of
S. aureus strains obtained from several US hospitals are methicillin-resistant, with some strains also being resistant to vancomycin and carbapenems
[81][126]. MDR microorganisms are frequently linked to nosocomial infection. Some MDR bacteria, on the other hand, have become common sources of community-acquired illnesses. This is a significant breakthrough since community-wide MDR bacteria dissemination leads to a significant rise in the population at risk and causes an increase in the number of MDR-bacteria-related diseases. When the incidence of resistance patterns in bacteria causing community-acquired infections exceeds a certain threshold, broad-spectrum antibacterial and/or combination antibacterial therapy is indicated for the empiric treatment of community-acquired disorders. Efforts to combat drug-resistant diseases are being hampered by the sluggish discovery of new antibiotics. It is anticipated that there will be no effective antibiotics available by 2050 if no new antibiotics are discovered
[82][127]. Due to the lack of effective antibiotics against MDR bacteria, developing nanoparticles has been used as a substitute. It has also been observed that bimetallic NPs are efficient against bacteria, including MDR bacteria
[83][128]. Several studies have shown bimetallic NPs to be effective against MDR bacteria. When monometallic counterparts were joined to form bimetallic NPs, the antibacterial activity was increased
[84][129].
In a study, Wang et al., reported that Au NPs and mercaptophenylboronic acid (MBA) are incapable of acting as antibiotics separately. However, when MBA was coupled with Au NPs, the Au–MBA NPs showed significant antibacterial activity against Gram-positive MDR clinical isolates (e.g., MDR
Staphyloccocus aureus and MDR
Staphyloccocus epidermidis)
[85][130]. In a similar study, Zhao and his collaborators synthesized bimetallic NPs by combining two salt solutions in an aqueous phase and reducing them with sodium borohydride. Monometallic NPs were also synthesized by the same method as the corresponding salt for comparison. The antibacterial NPs’ MIC (minimal inhibitory concentration) against
E. coli and
S. aureus were determined. Out of the nine different synthesized bimetallic NPs screened, two types of bimetallic NPs, namely the AuRh and AuRu NPs, showed MICs of 7 and 20 µg/mL, respectively, against
E. coli and
S. aureus. All of these bimetallic NPs were ineffective against
S. aureus, with the MIC for
S. aureus exceeding 128 µg/mL
[86][131]. Kumar and his colleagues developed carbohydrate-coated bimetallic Au–Ag NPs, which were more effective against MDR strains than their monometallic counterparts (i.e., Ag NPs and Au NPs). The Au–Ag NPs were significantly more capable against Gram-negative MDR
E. coli and
Enterobacter cloacae than standard antibiotics. An in vivo study also exhibited that bimetallic Au–Ag NPs were almost 11,000 times more effective than Gentamicin at killing MDR MRSA infecting mice skin wounds. The Au–Ag NPs could heal and regenerate the infected wounds faster and without scarring. The in vivo results showed that Au–Ag NPs are an effective antibacterial agent against MDR strains with no adverse side effects
[87][82].
Other forms of Au–Ag bimetallic NPs have been investigated and their antibacterial activity studied, although mostly as coating agents rather than as a delivery method
[88][132].
6. Gold, Silver, and Gold–Silver Nanomaterials for Wound Healing
Wound healing is a complex biological process involving a series of cellular and molecular interactions targeted at repairing the injured tissue and restoring its protective function. The wound healing process occurs simultaneously in four different steps: hemostasis, inflammation, proliferation, and remodeling, all of which occur simultaneously. Various medications are now available on the market that can aid with wound healing. For wound healing, drugs that target blood coagulation, inflammatory reactions, platelet function, and cell proliferation are often employed. Glucocorticoids, nonsteroidal anti-inflammatory drugs, and chemotherapeutic agents are examples of these medications
[87][89][90][82,133,134].
Bimetallic Au–Ag NPs are attractive candidates for wound dressing integration due to their high antibacterial potential and reduced toxicity profile compared to monometallic silver and gold NPs. According to Mârza et al., the antibacterial characteristics of silver can impact the healing process of skin regeneration, and in the meantime, the antibacterial properties of silver can assist an open wound by avoiding bacterial infection
[91][135].
The skin healing and regeneration ability of bioactive glass with spherical gold nanocages in Vaseline ointments were examined in vivo in this researchtudy, which compared bioactive-glass–Vaseline and bioactive glass with spherical-gold–Vaseline ointments. Because the spherical gold nanocages were supported by silver, they had a high antibacterial activity. The findings indicated that the presence of silver in a wound affected the healing process. Jiang et al., developed a green synthetic method for bimetallic Au–Ag NPs without using any surfactants and stabilizers. When E. coli and Au ions were retained in the same solution, Au nanoparticles were formed first by raising the pH. Core–shell Au–Ag nanoparticles were generated in an ordered manner when the Ag ions combined.