Manganese oxides are considered an essential component of natural geochemical barriers due to their redox and sorptive reactivity towards essential and potentially toxic trace elements. Despite the perception that they are in a relatively stable phase, microorganisms can actively alter the prevailing conditions in their microenvironment and initiate the dissolution of minerals, a process that is governed by various direct (enzymatic) or indirect mechanisms. Microorganisms are also capable of precipitating the bioavailable manganese ions via redox transformations into biogenic minerals, including manganese oxides (e.g., low-crystalline birnessite) or oxalates. Microbially mediated transformation influences the (bio)geochemistry of manganese and also the environmental chemistry of elements intimately associated with its oxides. Therefore, the biodeterioration of manganese-bearing phases and the subsequent biologically induced precipitation of new biogenic minerals may inevitably and severely impact the environment.
- manganese
- biotransformation
- microorganisms
- manganese oxides
- sorption
1. Introduction
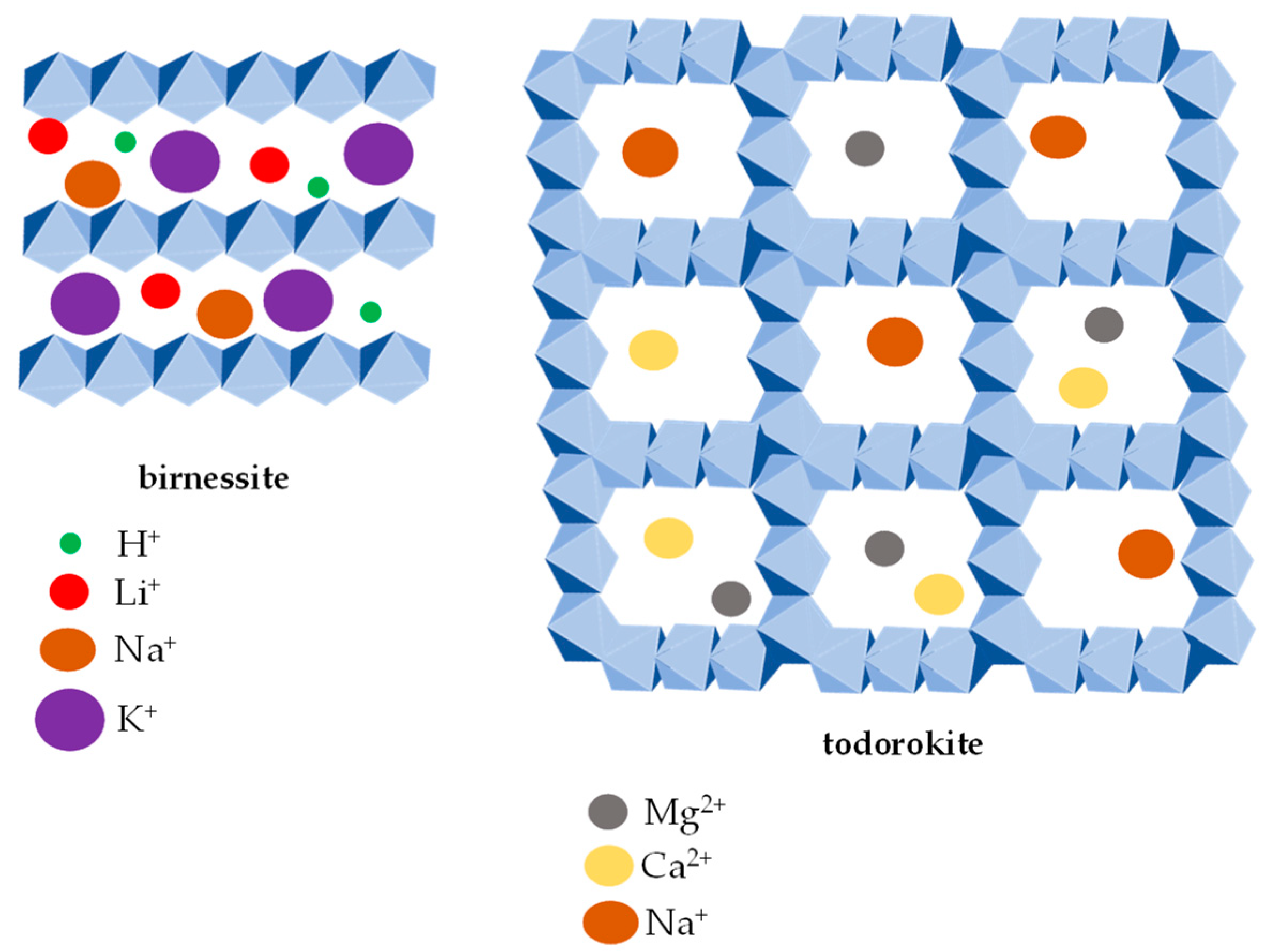
2. Manganese in Soils
| Mineral | PZC | Chemical Formula | Structure |
|---|---|---|---|
| Birnessite | 1.18–2.8 [42,43,44][42][43][44] | Na7Ca3Mn7O14 2.8H2O | layer |
| Cryptomelane | 1.98–2.1 [42,43][42][43] | Kx(MnIIIMnIV)8O16 (x = 1.3–1.5) | tunnel |
| Hollandite (α-MnO2) | 4.6 [45] | Bax(MnIIIMnIV)8O16(x ˂ 1) | tunnel |
| Lithiophorite | 6.9 [44] | LiAl2(MnIIIMnIV)3O6(OH)6 | layer |
| Todorokite | 3.2–3.98 [42,43,44][42][43][44] | (Ca, Na, K)0.3–0.5(MnIIIMnIV)6O12 3.5 H2O | tunnel |
| Vernadite (δ-MnO2) | 2.8–3.1 [46,47][46][47] | MnO2 | layer |
References
- Perel’man, A.I. Geochemical barriers: Theory and practical applications. Appl. Geochem. 1986, 1, 669–680.
- Khaustov, A.; Redina, M. Geochemical barriers as structural components of the geochemical systems evolution. E3S Web. Conf. 2019, 98, 01026.
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328.
- Allard, S.; Gutierrez, L.; Fontaine, C.; Croué, J.-P.; Gallard, H. Organic matter interactions with natural manganese oxide and synthetic birnessite. Sci. Total Environ. 2017, 583, 487–495.
- Bernard, S.; Chazal, P.; Mazet, M. Removal of organic compounds by adsorption on pyrolusite (β-MnO2). Water Res. 1997, 31, 1216–1222.
- Bin Yousaf, A.; Zavahir, S.; Zeb, A.; Michalcova, A.; Kasak, P. Nanostructural synergism as MnNC channels in manganese (IV) oxide and fluffy g-C3N4 layered composite with exceptional catalytic capabilities. J. Colloid. Interface Sci. 2022, 610, 258–270.
- Remucal, C.K.; Ginder-Vogel, M. A critical review of the reactivity of manganese oxides with organic contaminants. Environ. Sci. Process. Impacts 2014, 16, 1247–1266.
- Dikicioglu, D.; Oliver, S.G. Extension of the yeast metabolic model to include iron metabolism and its use to estimate global levels of iron-recruiting enzyme abundance from cofactor requirements. Biotechnol. Bioeng. 2019, 116, 610–621.
- Ijaz, A.; Mumtaz, M.Z.; Wang, X.; Ahmad, M.; Saqib, M.; Maqbool, H.; Zaheer, A.; Wang, W.; Mustafa, A. Insights Into Manganese Solubilizing Bacillus spp. for Improving Plant Growth and Manganese Uptake in Maize. Front. Plant Sci. 2021, 12, 719504.
- Hazen, R.; Papineau, D.; Bleeker, W.; Downs, R.; Ferry, J.; McCoy, T.; Sverjensky, D.; Yang, H. Mineral evolution. Am. Mineral. 2008, 93, 1693–1720.
- Dong, H.; Huang, L.; Zhao, L.; Zeng, Q.; Liu, X.; Sheng, Y.; Shi, L.; Wu, G.; Jiang, H.; Li, F.; et al. A critical review of mineral–microbe interaction and co-evolution: Mechanisms and applications. Natl. Sci. Rev. 2022, 9, nwac128.
- Ribeiro, I.D.A.; Volpiano, C.G.; Vargas, L.K.; Granada, C.E.; Lisboa, B.B.; Passaglia, L.M.P. Use of Mineral Weathering Bacteria to Enhance Nutrient Availability in Crops: A Review. Front. Plant Sci. 2020, 11, 590774.
- Duckworth, O.W.; Bargar, J.R.; Sposito, G. Coupled biogeochemical cycling of iron and manganese as mediated by microbial siderophores. BioMetals 2009, 22, 605–613.
- Kolenčík, M.; Urík, M.; Štubňa, J. Heterotrophic leaching and its application in biohydrometallurgy. Chem. Listy 2014, 108, 1040–1045.
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643.
- Hartshorne, R.S.; Reardon, C.L.; Ross, D.; Nuester, J.; Clarke, T.A.; Gates, A.J.; Mills, P.C.; Fredrickson, J.K.; Zachara, J.M.; Shi, L.; et al. Characterization of an electron conduit between bacteria and the extracellular environment. Proc. Natl. Acad. Sci. USA 2009, 106, 22169.
- Farkas, B.; Bujdoš, M.; Polák, F.; Matulová, M.; Cesnek, M.; Duborská, E.; Zvěřina, O.; Kim, H.; Danko, M.; Kisová, Z.; et al. Bioleaching of Manganese Oxides at Different Oxidation States by Filamentous Fungus Aspergillus niger. J. Fungi 2021, 7, 808.
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49.
- Farkas, B.; Urík, M.; Matúš, P. Manganese biotransformation by microorganisms. Chem. Listy 2020, 114, 841–846.
- Hagarová, I. Utilization of biosurfactants in remediation of environmental media contaminated with heavy metals. Chem. Listy 2015, 109, 431–436.
- Hagarová, I. Extractions Complying With the Principles of Green Chemistry Used in Trace Analysis of Metals. Chem. Listy 2015, 109, 269–275.
- Attanayake, C.P.; Kumaragamage, D.; Amarawansha, G.; Hettiarachchi, G.M.; Indraratne, S.P.; Goltz, D.M. Phosphorus Release and Speciation in Manganese (IV) Oxide and Zeolite-Amended Flooded Soils. Environ. Sci. Technol. 2022, 56, 8082–8093.
- Wu, K.; Li, Y.; Liu, T.; Huang, Q.; Yang, S.; Wang, W.; Jin, P. The simultaneous adsorption of nitrate and phosphate by an organic-modified aluminum-manganese bimetal oxide: Adsorption properties and mechanisms. Appl. Surf. Sci. 2019, 478, 539–551.
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300.
- Corpas, F.J.; Barroso, J.B.; Palma, J.M.; Rodriguez-Ruiz, M. Plant peroxisomes: A nitro-oxidative cocktail. Redox. Biol. 2017, 11, 535–542.
- Dreyer, M.; Wichmann, M.; Rischen, M.; Görlach, B.M.; Ehmke, A.; Pitann, B.; Mühling, K.H. Ammonium-driven nitrification plays a key role in increasing Mn availability in calcareous soils. J. Plant Nutr. Soil Sci. 2020, 183, 550.
- Huang, Y.L.; Yang, S.; Long, G.X.; Zhao, Z.K.; Li, X.F.; Gu, M.H. Manganese Toxicity in Sugarcane Plantlets Grown on Acidic Soils of Southern China. PLoS ONE 2016, 11, e0148956.
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447.
- Vodyanitskii, Y.N. Mineralogy and geochemistry of manganese: A review of publications. Eurasian. Soil Sci. 2009, 42, 1170–1178.
- Chukhrov, F.V.; Gorshkov, A.I.; Rudnitskaya, E.S.; Beresovskaya, V.V.; Sivtsov, A.V. Manganese Minerals in Clays: A Review. Clays Clay Miner. 1980, 28, 346–354.
- Robson, A.D. Manganese in Soils and Plants—An Overview. In Manganese in Soils and Plants, Proceedings of the International Symposium on ‘Manganese in Soils and Plants’ Held at the Waite Agricultural Research Institute, The University of Adelaide, Glen Osmond, Australia, 22–26 August 1988; as an Australian Bicentennial Event; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 329–333.
- Bowen, H. Environmental Chemistry of Elements; Acedemic Press: New York, NY, USA, 1979.
- Adriano, D.C. Manganese. In Trace Elements in the Terrestrial Environment; Adriano, D.C., Ed.; Springer: New York, NY, USA, 1986; pp. 263–297.
- Cornell, R.M.; Schwertmann, U. Cation Substitution. In The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Cornell, R.M., Schwertmann, U., Eds.; Wiley: New York, NY, USA, 2006; pp. 39–58.
- Gilkes, R.J.; McKenzie, R.M. Geochemistry and Mineralogy of Manganese in Soils. In Manganese in Soils and Plants, Proceedings of the International Symposium on ‘Manganese in Soils and Plants’ Held at the Waite Agricultural Research Institute, The University of Adelaide, Glen Osmond, Australia, 22–26 August 1988; as an Australian Bicentennial Event; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 23–35.
- Jones, M.E.; LaCroix, R.E.; Zeigler, J.; Ying, S.C.; Nico, P.S.; Keiluweit, M. Enzymes, Manganese, or Iron? Drivers of Oxidative Organic Matter Decomposition in Soils. Environ. Sci. Technol. 2020, 54, 14114–14123.
- Nicholson, K.; Hein, J.R.; Bühn, B.; Dasgupta, S. Manganese Mineralization: Geochemistry And Mineralogy of Terrestrial And Marine Deposits; Geological Society: London, UK, 1997; p. 370.
- Kabata-Pendias, A. Trace Elements in Soils and Plants, Fourth Edition; Taylor & Francis: Abingdon, UK, 2010.
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in Crop Production. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2002; Volume 77, pp. 185–268.
- Howe, P.D.; Malcolm, H.M.; Dobson, S. Manganese and Its Compounds: Environmental Aspects; World Health Organization: Geneva, Switzerland, 2004; p. 63.
- Tebo, B.M.; He, L.M. Microbially Mediated Oxidative Precipitation Reactions. ACS Symp. Ser. 1999, 715, 393–414.
- Tan, W.; Lu, S.; Liu, F.; Feng, X.; He, J.; Koopal, L. Determination of the point-of-zero charge of manganese oxides with different methods including an improved salt titration method. Soil. Sci. 2008, 173, 277–286.
- Feng, X.H.; Zhai, L.M.; Tan, W.F.; Liu, F.; He, J.Z. Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals. Environ. Pollut. 2007, 147, 366–373.
- Kim, J.G.; Dixon, J.B.; Chusuei, C.C.; Deng, Y. Oxidation of Chromium (III) to (VI) by Manganese Oxides. Soil Sci. Soc. Am. J. 2002, 66, 306–315.
- Healy, T.W.; Herring, A.P.; Fuerstenau, D.W. The effect of crystal structure on the surface properties of a series of manganese dioxides. J. Colloid. Interface Sci. 1966, 21, 435–444.
- Zhang, S.; Chen, S.; Liu, F.; Li, J.; Liang, X.; Chu, S.; Xiang, Q.; Huang, C.; Yin, H. Effects of Mn average oxidation state on the oxidation behaviors of As (III) and Cr (III) by vernadite. Appl. Geochem. 2018, 94, 35–45.
- Stumm, W. Chemistry of the Solid-Water Interface: Processes at the Mineral-Water and Particle-Water Interface in Natural Systems; Wiley: Chichester, UK, 1992; p. 448.
- Mellin, T.A.; Lei, G. Stabilization of 10A-manganates by interlayer cations and hydrotemral treatment: Implications for the mineralogy of marine manganese concentrations. Mar. Geol. 1993, 115, 67–83.
- Burns, R.G.; Burns, V.M.; Stockman, H.W. The todorkite-buserite problem: Further considerations. Am. Mineral. 1985, 70, 205–208.
- Mc Kenzie, R.M. Manganese Oxides and Hydroxides. In Minerals in Soil Environments; Soil Science Society of America: Madison, WI, USA, 1989; pp. 439–465.
- Cui, H.-J.; Feng, X.-H.; He, J.-Z.; Tan, W.-F.; Liu, F. Effects of reaction conditions on the formation of todorokite at atmospheric pressure. Clays Clay Miner. 2006, 54, 605–615.
- Cui, H.; Liu, X.; Tan, W.; Feng, X.; Liu, F.; Ruan, H.D. Influence of Mn (III) availability on the phase transformation from layered buserite to tunnel-structured todorokite. Clays Clay Miner. 2008, 56, 397–403.
- Negra, C.; Ross, D.S.; Lanzirotti, A. Oxidizing Behavior of Soil Manganese. Soil Sci. Soc. Am. J. 2005, 69, 87–95.
- Marafatto, F.F.; Strader, M.L.; Gonzalez-Holguera, J.; Schwartzberg, A.; Gilbert, B.; Peña, J. Rate and mechanism of the photoreduction of birnessite (MnO2) nanosheets. Proc. Natl. Acad. Sci. USA 2015, 112, 4600–4605.
- Golden, D.C.; Chen, C.C.; Dixon, J.B. Transformation of Birnessite to Buserite, Todorokite, and Manganite under Mild Hydrothermal Treatment. Clays Clay Miner. 1987, 35, 271–280.
- Liu, J.; Makwana, V.; Cai, J.; Suib, S.L.; Aindow, M. Effects of Alkali Metal and Ammonium Cation Templates on Nanofibrous Cryptomelane-type Manganese Oxide Octahedral Molecular Sieves (OMS-2). J. Phys. Chem. B 2003, 107, 9185–9194.
