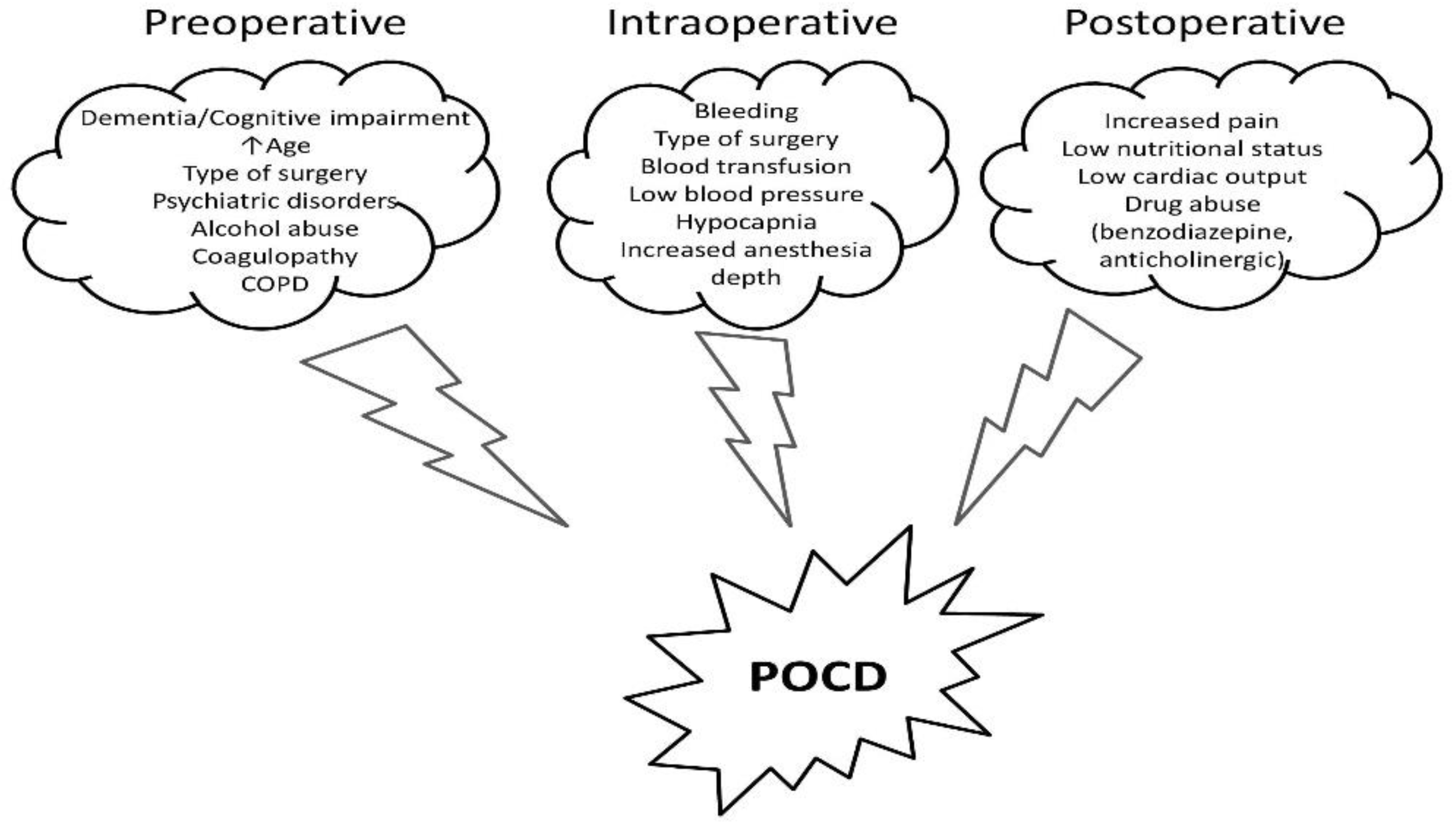
Postoperative cognitive dysfunction (POCD) represents a decreased cognitive performance in patients undergoing general anesthesia for major surgery. Since liver cirrhosis is associated with high mortality and morbidity rates, cirrhotic patients also assemble many risk factors for POCD. Therefore, preserving cognition after major surgery is a priority, especially in this group of patients. POCD is roughly defined as decreased cognitive performance in patients undergoing anesthesia. Various risk factors, including increased age, baseline cognition level, depth of anesthesia, or postoperative pain level, were described to be involved in the development of POCD. In the cirrhotic patient, POCD is described as a “more than expected” decline in cognitive function. The judicious use of anesthetic agents and techniques, the monitoring of the depth of anesthesia, and the application of ERAS protocols may prove to be advantageous in this setting. However, specific and targeted therapies for POCD are lacking.
- postoperative cognitive dysfunction
- cirrhosis
- hepatic encephalopathy
1. Introduction
2. Perioperative Cognitive Dysfunction Management in Cirrhotic Patients
With the increasing prevalence of chronic liver disease, up to 10% of cirrhotic patients may have non-transplant surgery within the last two years of their lives [21]. Depending on the degree of liver disease, perioperative mortality is generally 2–10 times higher in individuals with cirrhosis than in those without [22,23][22][23]. In cases of decompensated cirrhosis, general anesthesia and surgery might result in severe morbidity and significant perioperative mortality. The most significant clinical tools in identifying cirrhotic patients at risk for surgery and anesthesia, such as the MELD score, do not include, however, the presence of perioperative cognitive dysfunction, which can lead to prolonged ICU stay and, additionally, increase mortality [24,25][24][25]. Hemodynamic abnormalities, which are more obvious than all others, could occur as an inappropriate response to surgical stress due to the minimal hepatic reserve and the systemic disturbances brought on by liver dysfunction [24]. Furthermore, inappropriate blood flow to the brain along with disturbed liver metabolism could aggravate a subclinical cognitive dysfunction. Thus, supplementary perioperative complex cognitive assessments should be implemented, along with therapeutic strategies to mitigate this potentially dangerous complication. The clinician’s efforts should focus on the three main critical care stages: preoperative, intraoperative, and postoperative period, in order to develop new innovative strategies to reduce cognitive impairment in patients with end-stage liver disease. The most important perioperative strategies are summarized in Figure 43.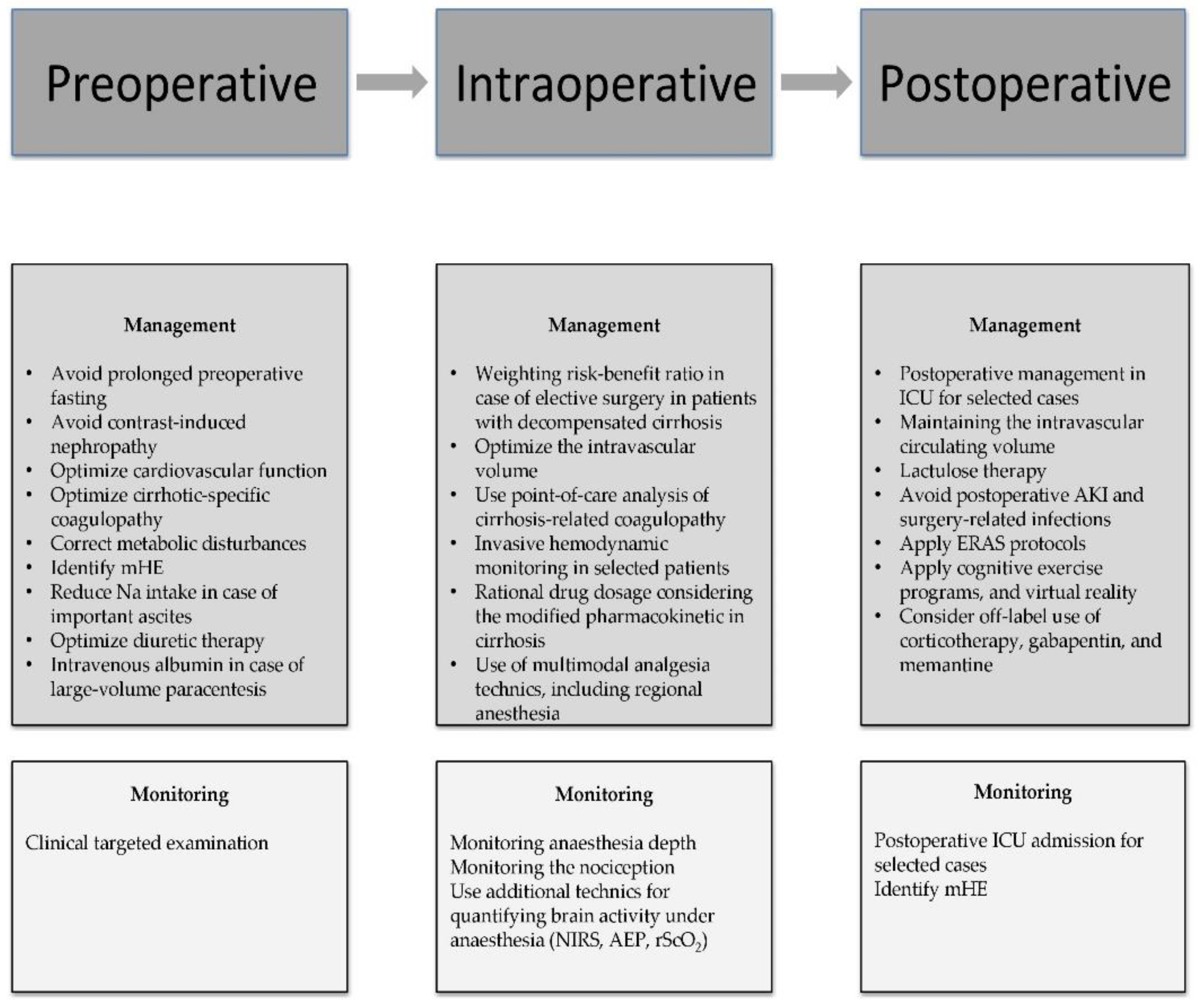
2.1. Preoperative Management of Cognitive Dysfunction in Cirrhotic Patients
According to the literature, age, ASA classification, and the degree of liver disease as mirrored by the MELD score are the three most significant predictors of mortality [25]. Nevertheless, emergency surgery has been linked to greater rates of morbidity and death rates than elective surgery in cirrhotic patients [24,26][24][26]. Even if no direct causality was observed between the degree of surgical emergency and cognitive decline, various perioperative complications such as hypotension, renal failure, and coagulation abnormalities could precipitate POCD in cirrhotic patients exposed to emergency surgery [27]. The most frequent indications for emergency surgery in cirrhotic patients are gallstones, abdominal wall hernia, small bowel, appendix, colorectal, or gastric surgery [24,28,29][24][28][29]. Thus, the clinician’s ability to predict and treat cirrhosis-associated comorbidities, regardless of the urgency grade, poses a central role in reducing the POCD rate.
2.2. Intraoperative Management of Cognitive Dysfunction in the Cirrhotic Patient
In addition to the significant risk of morbidity and mortality after anesthesia and surgery in cirrhosis, any preexistent cognitive deficit could influence postoperative care. The direct effect of anesthesia on the brain is still debatable [55,56][33][34]. Furthermore, specific novel biomarkers in cirrhosis could identify new predictive algorithms in patients at risk [14]. Elective interventions should be performed only in cases with compensated chronic liver disease [14,57][14][35]. Postponing elective surgery in patients with decompensated liver cirrhosis should be considered by weighting the risk-benefit ratio. Nevertheless, according to a study published by Friedman, there are several clinical conditions that contraindicate any elective surgery: fulminant hepatic failure, acute viral or alcoholic hepatitis, Child’s class C cirrhosis, severe coagulopathy, hypoxemia, cardiomyopathy, or acute renal failure [58][36]. Moreover, particular attention must be given to the neurological status of the patient and special measures taken in the perioperative period in order to minimize POCD, even if no consistent recommendation exists with regard to postponing an elective surgery based on the presence of HE or any other cognitive decline alone [59,60][37][38]. Intraoperative management poses a few challenges, such as optimization of the intravascular volume in the presence of ascites and peripheral edema. Goal-directed fluid therapy and avoiding overloading appear to be beneficial [1]. Blood products are routinely used in these cases and employment of point-of-care analysis of coagulopathy, such as ROTEM or TEG, proves to be an optimal approach [61][39]. Evaluating baseline cognitive function may prove useful in identifying patients at risk of developing POCD, as preoperative cognitive impairment is correlated with an increased probability of POCD [1]. Anesthesia and surgery-related risk factors for POCD refer to adequate analgesia or hypnosis, reduced blood flow to the brain, massive bleeding with an increased volume shift, and prolonged duration of the surgery [1]. Therefore, specific anesthetic strategies may be implemented in an attempt to minimize POCD. Intraoperative management becomes crucial in the health practitioners’ pursuit of maximizing postoperative brain health. Liver dysfunction associated with cirrhosis results in the alteration of all pharmacokinetic phases: absorption, distribution, and elimination [62][40]. Impaired liver function translates to increased portosystemic pressure, which increases the bioavailability of the drugs as a result of reduced first-pass metabolism. Diminished protein synthesis function, with lower serum albumin concentrations, as well as ascites formation and volume overload, explain the changes in the distribution phase in these patients [62][40]. Therefore, cirrhosis implies a higher volume of distribution for both highly protein-bound drugs and water-soluble drugs. The elimination phase is also subject to changes in liver disease, with different degrees of impairment of the various metabolizing enzymes, the cytochrome P450 system being more severely affected [62][40].2.3. Postoperative Management of Cognitive Dysfunction in the Cirrhotic Patient
Postoperative care may prove to be challenging in cirrhotic patients; maintaining cognitive status should be prioritized, even if no sufficient objective assessment tools are available for diagnosing the postoperative neurocognitive decline. Surgery and anesthesia might induce cirrhosis decompensation [60][38]. In addition to encephalopathy, other major complications could impact the outcome: ileus, infection, allergies to antibiotics, bleeding, coagulopathy, renal or respiratory failure, and new onset or worsening ascites [44,61,94][39][41][42]. Thus, cirrhotic patients, especially those in CTP-B and C class, should be managed in the ICU in the early postoperative period [61][39]. Assessment of bleeding risk, close monitoring of coagulation, and cautious fluid management are of marked importance. Cirrhotic patients exhibit a hyperdynamic status, with hypotension being regarded as a normal occurrence in these cases. The general volume overload associated with reduced intravascular volume in cirrhotic patients may warrant the use of invasive monitoring [60,95][38][43]. Lactic acidosis may occur not only in the setting of inadequate perfusion but also as a sign of liver decompensation [95][43]. Moreover, renal dysfunction following surgery in liver disease patients may be a result of either acute tubular necrosis or hepatorenal syndrome. Thus, maintaining the intravascular circulating volume is crucial in these patients [61][39]. Salt restriction is required as a preventive measure for acute kidney injury (AKI), HE, or ascites occurrence. Regarding coagulation, INR has been suggested to be a sensitive test in assessing hepatic synthesis and a useful marker of hepatic decompensation. Targeting normal values and overcorrection of INR through the administration of FFP in an otherwise hemostatic patient is not required [60,95][38][43].References
- Kotekar, N.; Shenkar, A.; Nagaraj, R. Postoperative Cognitive Dysfunction—Current Preventive Strategies. Clin. Interv. Aging 2018, 13, 2267–2273.
- Kapoor, I.; Prabhakar, H.; Mahajan, C. Postoperative Cognitive Dysfunction. Indian J. Crit. Care Med. 2019, 23, S162–S164.
- Moller, J.T.; Cluitmans, P.; Rasmussen, L.S.; Houx, P.; Rasmussen, H.; Canet, J.; Rabbitt, P.; Jolles, J.; Larsen, K.; Hanning, C.D.; et al. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet 1998, 351, 857–861.
- Silbert, B.S.; Evered, L.A.; Scott, D.A. Incidence of Postoperative Cognitive Dysfunction after General or Spinal Anaesthesia for Extracorporeal Shock Wave Lithotripsy. Br. J. Anaesth. 2014, 113, 784–791.
- Borozdina, A.; Porcella, L.; Bilotta, F. Postoperative Cognitive Dysfunction. In Essentials of Neuroanesthesia; Elsevier: Amsterdam, The Netherlands, 2017; pp. 661–667.
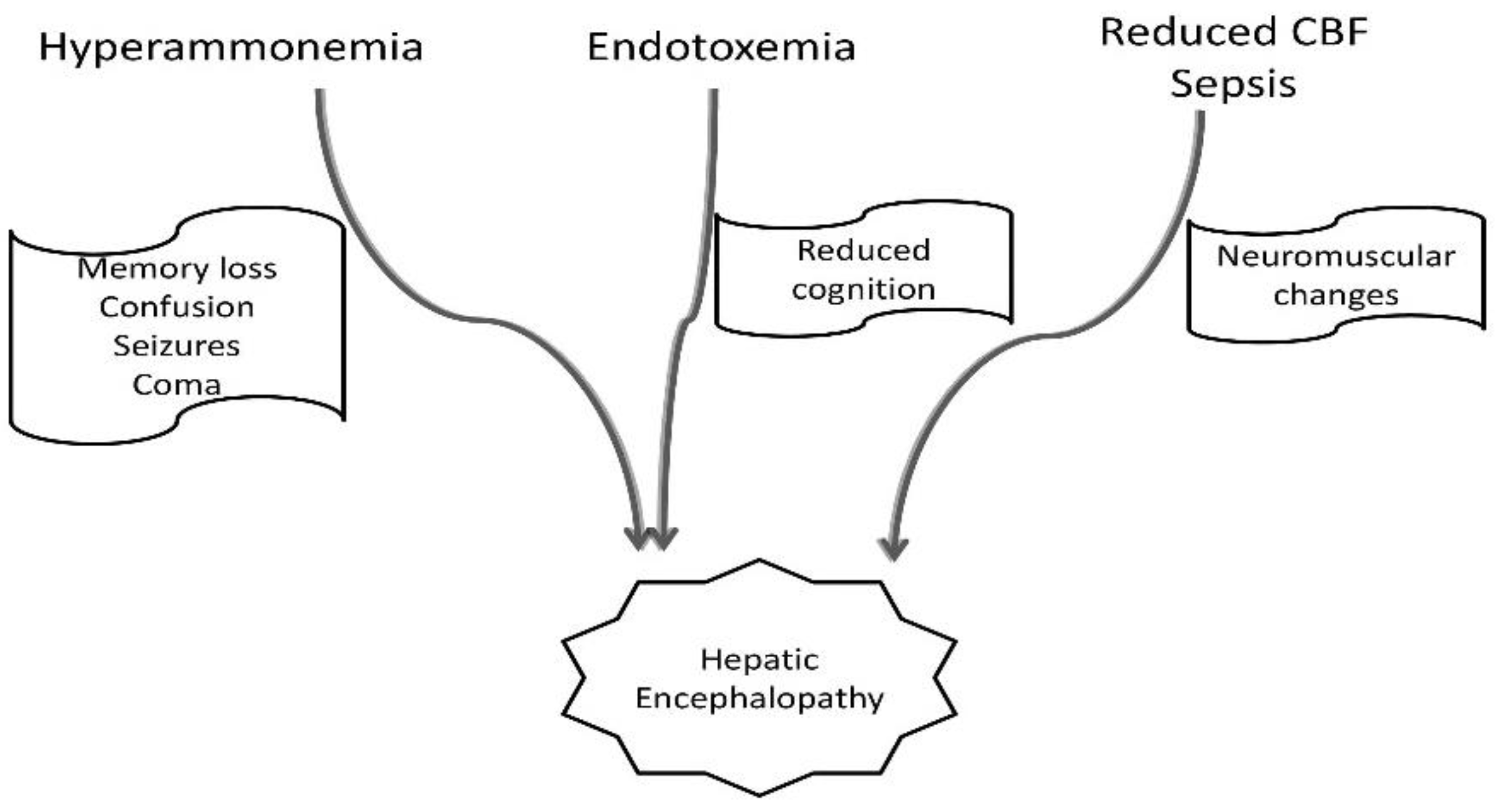
- Aldridge, D.R.; Tranah, E.J.; Shawcross, D.L. Pathogenesis of hepatic encephalopathy: Role of ammonia and systemic inflammation. J. Clin. Exp. Hepatol. 2015, 5 (Suppl. 1), S7–S20.
- Coltart, I.; Tranah, T.H.; Shawcross, D.L. Inflammation and Hepatic Encephalopathy. Arch. Biochem. Biophys. 2013, 536, 189–196.
- Bosoi, C.R.; Rose, C.F. Identifying the Direct Effects of Ammonia on the Brain. Metab. Brain Dis. 2009, 24, 95–102.
- Shawcross, D.L.; Shabbir, S.S.; Taylor, N.J.; Hughes, R.D. Ammonia and the Neutrophil in the Pathogenesis of Hepatic Encephalopathy in Cirrhosis. Hepatology 2010, 51, 1062–1069.
- Hadjihambi, A.; Arias, N.; Sheikh, M.; Jalan, R. Hepatic Encephalopathy: A Critical Current Review. Hepatol. Int. 2018, 12, 135–147.
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic Encephalopathy—Definition, Nomenclature, Diagnosis, and Quantification: Final Report of the Working Party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721.
- López-Franco, Ó.; Morin, J.-P.; Cortés-Sol, A.; Molina-Jiménez, T.; del Moral, D.I.; Flores-Muñoz, M.; Roldán-Roldán, G.; Juárez-Portilla, C.; Zepeda, R.C. Cognitive Impairment After Resolution of Hepatic Encephalopathy: A Systematic Review and Meta-Analysis. Front. Neurosci. 2021, 15, 579263.
- Isac, T.; Isac, S.; Ioanitescu, S.; Mihaly, E.; Tanasescu, M.-D.; Balan, D.; Tulin, A.; Iliescu, L. Dynamics of Serum A-fetoprotein in Viral Hepatitis C without Hepatocellular Carcinoma. Exp. Ther. Med. 2021, 22, 749.
- Isac, T.; Isac, S.; Rababoc, R.; Cotorogea, M.; Iliescu, L. Epigenetics in Inflammatory Liver Diseases: A Clinical Perspective (Review). Exp. Ther. Med. 2022, 23, 366.
- Ahluwalia, V.; Wade, J.B.; Heuman, D.M.; Hammeke, T.A.; Sanyal, A.J.; Sterling, R.K.; Stravitz, R.T.; Luketic, V.; Siddiqui, M.S.; Puri, P.; et al. Enhancement of Functional Connectivity, Working Memory and Inhibitory Control on Multi-Modal Brain MR Imaging with Rifaximin in Cirrhosis: Implications for the Gut-Liver-Brain Axis. Metab. Brain Dis. 2014, 29, 1017–1025.
- Rovira, A.; Córdoba, J.; Raguer, N.; Alonso, J. Magnetic Resonance Imaging Measurement of Brain Edema in Patients with Liver Disease: Resolution after Transplantation. Curr. Opin. Neurol. 2002, 15, 731–737.
- Zhang, L.J.; Zhong, J.; Lu, G.M. Multimodality MR imaging findings of low-grade brain edema in hepatic encephalopathy. Am. J. Neuroradiol. 2013, 34, 707–715.
- Teperman, L.W. Impact of Pretransplant Hepatic Encephalopathy on Liver Posttransplantation Outcomes. Int. J. Hepatol. 2013, 2013, 952828.
- Ardizzone, G.; Arrigo, A.; Schellino, M.M.; Stratta, C.; Valzan, S.; Skurzak, S.; Andruetto, P.; Panio, A.; Ballaris, M.A.; Lavezzo, B.; et al. Neurological Complications of Liver Cirrhosis and Orthotopic Liver Transplant. Transplant. Proc. 2006, 38, 789–792.
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.P.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the Management of Patients with Decompensated Cirrhosis. J. Hepatol. 2018, 69, 406–460.
- Bleszynski, M.S.; Bressan, A.K.; Joos, E.; Morad Hameed, S.; Ball, C.G. Acute care and emergency general surgery in patients with chronic liver disease: How can we optimize perioperative care? A review of the literature. World J. Emerg. Surg. 2018, 13, 32.
- Rai, R.; Nagral, S.; Nagral, A. Surgery in a Patient with Liver Disease. J. Clin. Exp. Hepatol. 2012, 2, 238–246.
- Newman, K.L.; Johnson, K.M.; Cornia, P.B.; Wu, P.; Itani, K.; Ioannou, G.N. Perioperative Evaluation and Management of Patients with Cirrhosis: Risk Assessment, Surgical Outcomes, and Future Directions. Clin. Gastroenterol. Hepatol. 2020, 18, 2398–2414.
- Bhangui, P.; Laurent, A.; Amathieu, R.; Azoulay, D. Assessment of Risk for Non-Hepatic Surgery in Cirrhotic Patients. J. Hepatol. 2012, 57, 874–884.
- Teh, S.H.; Nagorney, D.M.; Stevens, S.R.; Offord, K.P.; Therneau, T.M.; Plevak, D.J.; Talwalkar, J.A.; Kim, W.R.; Kamath, P.S. Risk Factors for Mortality After Surgery in Patients with Cirrhosis. Gastroenterology 2007, 132, 1261–1269.
- Csikesz, N.G.; Nguyen, L.N.; Tseng, J.F.; Shah, S.A. Nationwide Volume and Mortality after Elective Surgery in Cirrhotic Patients. J. Am. Coll. Surg. 2009, 208, 96–103.
- Abbas, N.; Makker, J.; Abbas, H.; Balar, B. Perioperative Care of Patients with Liver Cirrhosis: A Review. Health Serv. Insights 2017, 10, 1178632917691270.
- Wetterkamp, M.; van Beekum, C.J.; Willis, M.A.; Glowka, T.R.; Manekeller, S.; Fimmers, R.; Praktiknjo, M.; Chang, J.; Kalff, J.C.; Vilz, T.O. Risk Factors for Postoperative Morbidity and Mortality after Small Bowel Surgery in Patients with Cirrhotic Liver Disease-A Retrospective Analysis of 76 Cases in a Tertiary Center. Biology 2020, 9, 349.
- Andraus, W.; Pinheiro, R.S.; Lai, Q.; Haddad, L.B.P.; Nacif, L.S.; D’Albuquerque, L.A.C.; Lerut, J. Abdominal Wall Hernia in Cirrhotic Patients: Emergency Surgery Results in Higher Morbidity and Mortality Visceral and General Surgery. BMC Surg. 2015, 15, 65.
- Telem, D.A.; Schiano, T.; Goldstone, R.; Han, D.K.; Buch, K.E.; Chin, E.H.; Nguyen, S.Q.; Divino, C.M. Factors That Predict Outcome of Abdominal Operations in Patients with Advanced Cirrhosis. Clin. Gastroenterol. Hepatol. 2010, 8, 451–457.
- Perkins, L.; Jeffries, M.; Patel, T. Utility of Preoperative Scores for Predicting Morbidity After Cholecystectomy in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2004, 2, 1123–1128.
- Northup, P.G.; Wanamaker, R.C.; Lee, V.D.; Adams, R.B.; Berg, C.L. Model for End-Stage Liver Disease (MELD) Predicts Nontransplant Surgical Mortality in Patients with Cirrhosis. Ann. Surg. 2005, 242, 244–251.
- Isac, S.; Pavel, B.; Dobre, M.; Milanesi, E.; Matache, I.-M.; Paun, R.-M.; Klimko, A.; Iesanu, M.I.; Droc, G.; Zagrean, A.-M. Does a Single Exposure to General Anesthesia Have a Cumulative Effect on the Developing Brain after Mild Perinatal Asphyxia? Life 2022, 12, 1568.
- Liu, X.; Ji, J.; Zhao, G.Q. General Anesthesia Affecting on Developing Brain: Evidence from Animal to Clinical Research. J. Anesth. 2020, 34, 765–772.
- Maze, M.; Bass, N.M. Anaesthesia and the Hepatobiliary System. In Anesthesia, 5th ed.; Miller, R.D., Ed.; Churchill Livingstone: London, UK, 2000; ISBN 9780443079788.
- Friedman, L.S. The Risk of Surgery in Patients with Liver Disease. Hepatology 1999, 29, 1617–1623.
- Rahimzadeh, P.; Safari, S.; Reza Faiz, S.H.; Alavian, S.M. Anesthesia for Patients with Liver Disease. Hepat. Mon. 2014, 14, e19881.
- Vaja, R.; McNicol, L.; Sisley, I. Anaesthesia for Patients with Liver Disease. Contin. Educ. Anaesth. Crit. Care Pain 2010, 10, 15–19.
- Lopez-Delgado, J.C.; Ballus, J.; Esteve, F.; Betancur-Zambrano, N.L.; Corral-Velez, V.; Mañez, R.; Betbese, A.J.; Roncal, J.A.; Javierre, C. Outcomes of Abdominal Surgery in Patients with Liver Cirrhosis. World J. Gastroenterol. 2016, 22, 2657–2667.
- Mcclain, R.L.; Ramakrishna, H.; Iii, S.A.; Cartwright, J.A.; Phar, L.G.W.; Pai, S.-L.; Rodrigues, E.S.; Martin, A.K.; Shine, T.S. Anesthetic Pharmacology and Perioperative Considerations for the End Stage Liver Disease Patient. Curr. Clin. Pharmacol. 2015, 10, 35–46.
- Millwala, F.; Nguyen, G.C.; Thuluvath, P.J. Outcomes of Patients with Cirrhosis Undergoing Non-Hepatic Surgery: Risk Assessment and Management. World J. Gastroenterol. 2007, 13, 4056–4063.
- Chatzizacharias, N.A.; Bradley, J.A.; Harper, S.; Butler, A.; Jah, A.; Huguet, E.; Praseedom, R.K.; Allison, M.; Gibbs, P. Successful Surgical Management of Ruptured Umbilical Hernias in Cirrhotic Patients. World J. Gastroenterol. 2015, 21, 3109–3113.
- Harmouch, M.A.; Hobeika, M.J. Perioperative Management of the Cirrhotic Patient. In Common Problems in Acute Care Surgery; Moore, L.J., Turner, K.L., Todd, S.R., Eds.; Springer International Publishing: New York, NY, USA, 2017; ISBN 978-3-319-42790-4.
