Plastic pollution in the world is widespread and growing. The environment is swamped with nanoplastics (<100 nm), and the health consequences of these less visible pollutants are unknown. Furthermore, there is evidence that microplastics can release nanoplastics by digestive disintegration, implying that macroplastic exposure can cause direct and indirect disease via nanoplastics. Nanoplastics enters an organism through the respiratory and gastro-intestinal tract where they accumulate into the liver through blood circulation via absorption, or epidermal infiltration. It is stated that macroplastics can cause damage directly at the site of exposure, whereas nanoplastics can influence the liver, causing subsequent damage to other organs. Multi-organ dysfunction is brought on by liver changes, and nanoplastics can readily enter the gut-liver axis and disturb the gut microflora.
- nanoplastics
- hepatic glucose metabolism
- lipid peroxidation
- metabolic dysfunction
- gut-liver axis
1. Introduction
2. Pathways Involved in Liver Metabolism
NPs of diameter <100 nm have had particular attention in the science field and are commonly used in studies, because they are internalized by cells more than larger particles [31][25]. Evidence has indicated that the liver and kidneys are major accumulation sites, as well as vital organs for the metabolism and clearance of nanomaterials [32][26]. The accumulation of polystyrene nanoplastics (PS NPs) in the liver and kidneys of mice has been documented, and exposure to NPs induced evident morphological and functional changes in these two organs, suggesting the importance of investigating the impact of NPs on liver and renal cells [33,34,35][27][28][29]. The majority of total glucose disposal happens in insulin-independent tissues, with roughly 50% occurring in the brain and 25% occurring in the splanchnic region (liver and the gastrointestinal tissues) [36][30]. The release of glucose from the liver closely matches glucose use, which averages around 2.0 mg/kg/min [37][31]. The majority of glucose elimination in the body happens in muscle tissue after consuming a glucose-containing meal [38][32]. Glucose homeostasis is dependent on three interconnected processes: pancreatic insulin secretion, stimulation of glucose uptake by splanchnic (liver) and peripheral tissues, and inhibition of hepatic glucose output [39][33]. Adipose tissue accounts for only 4%–5% of total body glucose elimination, which plays a critical role in maintaining whole body glucose homeostasis [40][34]. Pathways involved in liver metabolism related to muscles and adipose tissue are also mentioned (Figure 1).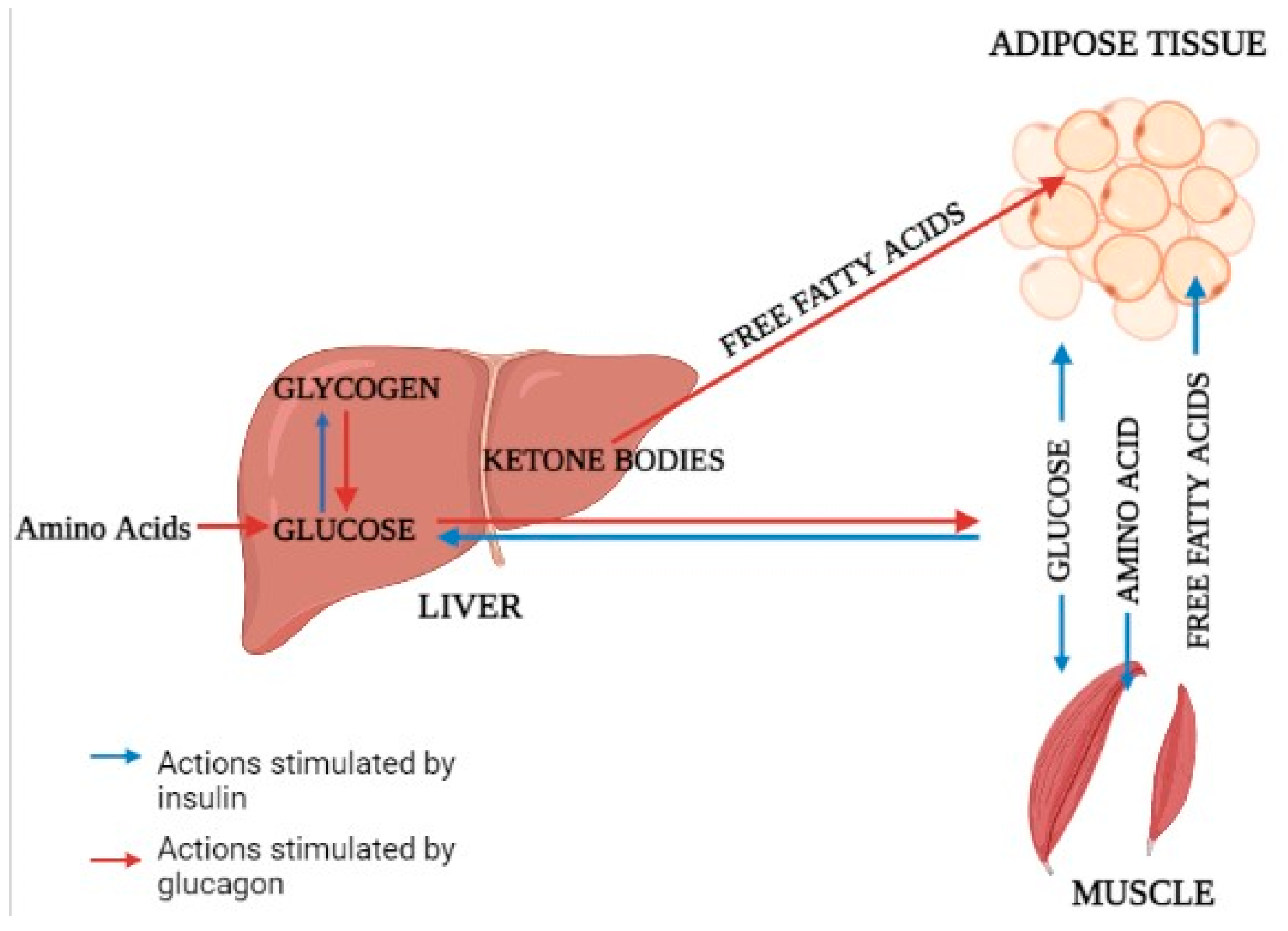
2.1. Glucose Metabolism
In humans, muscle is the principal location of glucose excretion. Once in the cell, glucose can be converted to glycogen or enter the glycolytic pathway [41][35]. Because obesity and diabetes are the two most prominent risk factors for the development of liver disorder, the presence of one or both of them can explain some of the peripheral insulin resistance IR [42,43][36][37]. Glucose disposal is reduced by about 50% in nondiabetic patients compared to normal people, a level comparable to type-2 diabetes mellitus (T2DM) [44][38]. The fact that there is no difference in glucose disposal between normal and overweight patients shows that the problem is not solely connected with improper glucose regulation and/or excess fat accumulation [45][39]. From fatty liver to nonalcoholic steatohepatitis (NASH), glucose utilization in muscle deteriorates progressively, and hyperinsulinemia are not secondary to a decrease in hepatic insulin extraction [46,47,48][40][41][42].2.2. Glucose Output
After an overnight fast, a healthy person’s liver produces glucose at a rate sufficient to meet the needs of the brain [51][43]. The variability of basal human glucose output (HGO) in nondiabetic persons is mostly determined by the amount of lean body mass and the degree of peripheral insulin use [52,53,54][44][45][46]. HGO is suppressed by insulin released into the portal vein following glucose or meal consumption. Hepatic IR occurs when the liver fails to detect this signal [54][46]. The glucose produced by the liver can come from glycogenolysis or gluconeogenesis [55][47]. Insulin suppresses HGO either directly through the hepatic insulin receptor or indirectly by reduced production of gluconeogenic substrates (e.g., alanine, lactate, glycerol, and free fatty acids) in both muscle and liver [56,57][48][49].2.3. Lipolysis and Lipid Oxidation
Aside from muscle and liver, adipose tissue is the third metabolically significant site of insulin action. Whereas insulin-stimulated glucose disposal in fat tissue is minor in comparison to that in muscle, regulation of lipolysis with subsequent release of glycerol and free fatty acids (FFAs) into the bloodstream has significant implications for glucose homeostasis [40,58][34][50]. Increased availability and utilization of FFAs aid in the development of skeletal muscle IR by inhibiting substrate oxidation competitively [59][51]. According to recent 1H nuclear magnetic resonance studies, an increase in intracellular fatty acid metabolites impairs IRS-1 tyrosine phosphorylation, resulting in decreased PI3-kinase activity and glucose transport [60][52]. Free fatty acids (FFAs) stimulate key enzymes and provide energy for gluconeogenesis, while glycerol released during triglyceride hydrolysis acts as a gluconeogenic substrate.2.4. Fatty Acid Metabolism and Energy Supply
The Krebs cycle oxidizes acetyl-coenzyme A (CoA), resulting in reduced forms of nicotinamide adenine dinucleotide (NADH) and reduced flavine-adenine dinucleotide, which transport electrons to the MRC [64][53]. AMPK promotes glucose and fatty acid oxidation while also activating PGC-1a [64,65][53][54]. PGC-1a interacts with peroxisome proliferator-activated receptor a (PPARa) to promote the production of numerous fatty acid-metabolizing enzymes, including carnitine palmitoyltransferase 1 (CPT1) and acyl-CoA dehydrogenases, hence enhancing fatty acid b-oxidation in mitochondria [66,67,68,69][55][56][57][58].2.5. Mitochondrial Damage
The TCA cycle is essential for cellular energy metabolism because it provides energy for cellular respiration [75][59]. In one study, NP-treated L02 cells had higher levels of some endogenous indicators of the TCA cycle, such as malate, and lower levels of others, such as fumarate. The effects of 80 nm NPs on mitochondrial activities and metabolic pathways in normal human hepatic (L02) cells were studied. NP did not cause widespread cell death. However, transmission electron microscopy analysis revealed that the NPs could enter the cells and cause mitochondrial damage, as evidenced by increased production of reactive oxygen species in the mitochondria, changes in the mitochondrial membrane potential, and suppression of mitochondrial respiration.2.6. Protein and Urea Metabolism
Among the three key dietary categories of protein, lipids, and carbohydrates, protein metabolism is crucial for the production of albumin and prothrombin as well as for the detoxification of ammonia. When these mechanisms are addressed in nutrition treatment for liver cirrhosis, they affect the development of hepatic encephalopathy and the hepatic functional reserve, leading to an imbalance in branched-chain amino acid (BCAA) insufficiency, reduced albumin synthesis, and elevated blood ammonia concentrations [82][60]. The primary reason of BCAA shortage in liver cirrhosis patients is an increase in ammonia metabolism in the skeletal muscles [83][61].2.7. Ethanol Metabolism
There are at least three different categories of ethanol’s metabolic effects: those brought on by changes in metabolite pools and cofactors brought on by the ethanol’s metabolism, those brought on by neuroendocrine issues brought on by intoxication, and those brought on directly by the pharmacological effects of ethanol on particular cells and processes. These numerous sorts of effects have varying degrees of contribution in practically every significant domain of metabolism. The apparent discrepancies about the metabolic effects of ethanol frequently result from variations in experimental setups that affect how much each of these components contributes [83,84][61][62].3. Impact of Nanoplastics on Liver Metabolism Causing Multi-Organ Dysfunction
The liver, a central metabolic organ, alters lipid metabolism in response to harsh environmental conditions [91][63]. Pathways involved in liver metabolism and neighboring organ dysfunction due to nanoplastics toxicity are presented (Figure 2). After being exposed to 100 ppm of PS-NPs, Lu et al. (2016) discovered that huge volumes of lipid droplets developed in the liver of the zebrafish Danio rerio [92][64]. As reported, it showed greater hepatic inflammation. According to the findings, dietary exposure to PS-NPs increased oxidative stress and interfered with lipid metabolism. Furthermore, after NP exposure, the crude lipid content of turbot Scophthalmus maximus and black rockfish Sebastes schlegelii livers increases dramatically [93,94][65][66]. An in vitro experiment revealed that NPs bind to apolipoprotein A-1 in fish serum, limiting lipid utilization [95,96][67][68]. According to a study, after PS NPs exposure, the liver TG and lipid content were considerably greater than in the control group. In fed fish, mRNA expression of the lipid synthesis-related gene fas and lipid transport-related genes such as cd36 and fatp1 increased considerably [97][69]. The mRNA expression of lipid catabolism-related genes such as ppar and aco increased initially and then declined as PS NP concentrations increased. PS NPs were found to boost the expression of genes involved in lipid production and catabolism in hepatocytes in the short term [98][70]. The trans-generational effect showed fabp10a expression in larval livers in a dose- and size-dependent manner. The 50 nm PS-NPs of 0.1ppm concentration raised fabp10a expression in the larval liver by 21.90%. These impacts’ potential mechanisms are dependent on their distribution and the formation of reactive oxygen species in the larvae. NPs also stimulate steroid hormone biosynthesis in zebrafish larvae, which may result in immune-related disorders [99,100][71][72].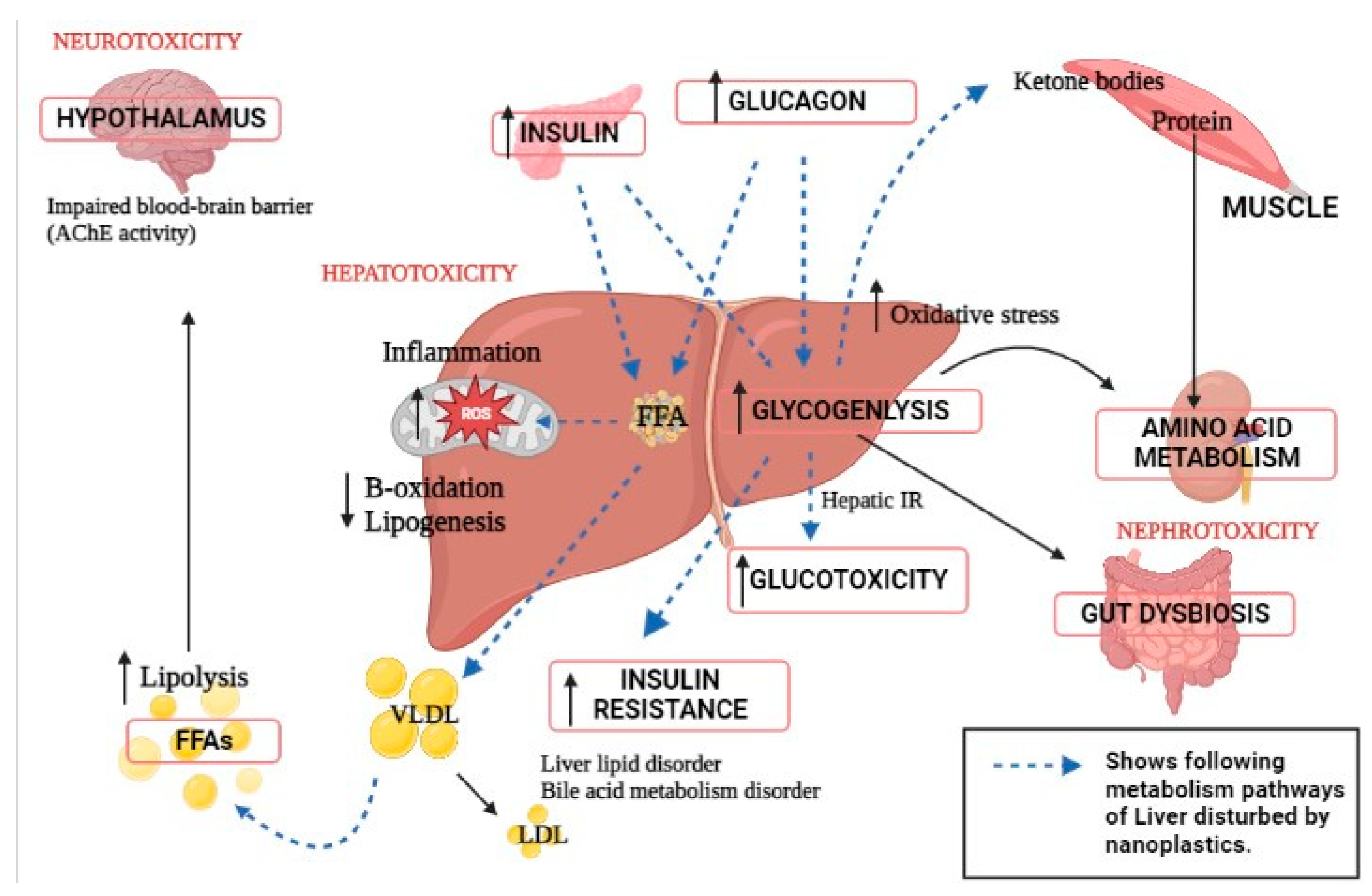
4. Alteration in Gut–Liver Axis Due to Nanoplastic Toxicity
The portal vein, which carries blood from the human digestive system to the liver and establishes the foundation for a strong bidirectional interaction between these two critical organs, connects the human liver and stomach. The liver participates via bile acid production and the facilitation of some parts of the human immune response via the portal vein, which transports different metabolites from the digestive tract to the liver [125][74]. There is evidence that gut microbiota and liver are related as a result of environmental pollutants. Mice were exposed to 1 mm polystyrene microplastics at a concentration of 10,000 g/L in one experiment. The main metabolic alterations in the liver after one week of microplastic exposure may be divided into two categories. The mice provided protection against the oxidative damage in the first section. In the second section, the mice’s gut–liver axis was disturbed, which increased in developing insulin resistance. After one week of exposure to microplastics, metabolites were altering, which raises the chance of developing diabetes. The regulation of these metabolites has significant effects on insulin resistance and the gut–liver axis metabolism. The gut–liver axis was linked to two elevated metabolites, 4-guanidinobutyric acid and CDP-choline. Mice showed significance for experiencing oxidative stress and building up resistance to it after one week of exposure to microplastics. The exposure to microplastics in mice disrupted the gut–liver axis based on information on intestinal microbiota and differently expressed metabolites [126][75].References
- Alabi, O.A.; Ologbonjaye, K.I.; Awosolu, O.; Alalade, O.E. Public and Environmental Health Effects of Plastic Wastes Disposal: A Review. J. Toxicol. Risk Assess. 2019, 5, 1–13.
- Geyer, R. Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Academic Press: Cambridge, MA, USA, 2020; pp. 13–32.
- Abbing, M.R. Plastic Soup: An Atlas of Ocean Pollution; Island Press: Washington DC, USA, 2019.
- Williams, M.; Gower, R.; Green, J.; Whitebread, E.; Lenkiewicz, Z.; Schröder, P. No Time to Waste: Tackling the Plastic Pollution Crisis before It’s Too Late; Tearfund: Teddington, UK, 2019.
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724.
- Li, Y.; Wang, Z.; Guan, B. Separation and Identification of Nanoplastics in Tap Water. Environ. Res. 2022, 204, 112134.
- Wang, H.; Shi, X.; Gao, Y.; Zhang, X.; Zhao, H.; Wang, L.; Zhang, X.; Chen, R. Polystyrene nanoplastics induce profound metabolic shift in human cells as revealed by integrated proteomic and metabolomic analysis. Environ. Int. 2022, 166, 107349.
- Lin, S.; Zhang, H.; Wang, C.; Su, X.-L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.-H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493.
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197.
- Lenard, N.R.; Berthoud, H.-R. Central and Peripheral Regulation of Food Intake and Physical Activity: Pathways and Genes. Obesity 2008, 16, S11–S22.
- Roseman, D.S.; Khan, T.; Rajas, F.; Jun, L.S.; Asrani, K.H.; Isaacs, C.; Farelli, J.D.; Subramanian, R.R. G6PC mRNA Therapy Positively Regulates Fasting Blood Glucose and Decreases Liver Abnormalities in a Mouse Model of Glycogen Storage Disease 1a. Mol. Ther. 2018, 26, 814–821.
- Hers, H.G.; Hue, L. Gluconeogenesis and related aspects of glycolysis. Annu. Rev. Biochem. 1983, 52, 617–653.
- Allen, M.S.; Bradford, B.J.; Oba, M. Board-invited review: The hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 2009, 87, 3317–3334.
- Wei, Y.; Li, B.; Xu, H.; Liang, M. Liver Metabolome and Proteome Response of Turbot (Scophthalmus maximus) to Lysine and Leucine in Free and Dipeptide Forms. Front. Mar. Sci. 2021, 8, 691404.
- Kroupina, K.; Bémeur, C.; Rose, C.F. Amino acids, ammonia, and hepatic encephalopathy. Anal. Biochem. 2022, 649, 114696.
- Matsumoto, S.; Haberle, J.; Kido, J.; Mitsubuchi, H.; Endo, F.; Nakamura, K. Urea cycle disorders—Update. J. Hum. Genet. 2019, 64, 833–847.
- Campbell, I. Liver: Metabolic functions. Anaesth. Intensiv. Care Med. 2006, 7, 51–54.
- Han, H.-S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.-H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218.
- Wang, X.; Li, H.; Chen, Y.; Meng, X.; Dieketseng, M.Y.; Wang, X.; Yan, S.; Wang, B.; Zhou, L.; Zheng, G. A neglected risk of nanoplastics as revealed by the promoted transformation of plasmid-borne ampicillin resistance gene by Escherichia coli. Environ. Microbiol. 2022, 24, 4946–4959.
- Wang, H.; Li, Z.; Chen, H.; Jin, J.; Zhang, P.; Shen, L.; Hu, S.; Liu, H. Metabolomic analysis reveals the impact of ketoprofen on carbon and nitrogen metabolism in rice (Oryza sativa L.) seedling leaves. Environ. Sci. Pollut. Res. 2022, 30, 21825–21837.
- Ahmad, F.; Wang, X.; Li, W. Toxico-Metabolomics of Engineered Nanomaterials: Progress and Challenges. Adv. Funct. Mater. 2019, 29, 1904268.
- Yin, K.; Wang, Y.; Zhao, H.; Wang, D.; Guo, M.; Mu, M.; Liu, Y.; Nie, X.; Li, B.; Li, J.; et al. A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci. Total Environ. 2021, 774, 145758.
- Xu, J.-L.; Lin, X.; Wang, J.J.; Gowen, A.A. A review of potential human health impacts of micro- and nanoplastics exposure. Sci. Total Environ. 2022, 851, 158111.
- He, Y.; Li, J.; Chen, J.; Miao, X.; Li, G.; He, Q.; Xu, H.; Li, H.; Wei, Y. Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Sci. Total Environ. 2020, 723, 138180.
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949.
- Li, X.; Wang, B.; Zhou, S.; Chen, W.; Chen, H.; Liang, S.; Zheng, L.; Yu, H.; Chu, R.; Wang, M.; et al. Surface chemistry governs the sub-organ transfer, clearance and toxicity of functional gold nanoparticles in the liver and kidney. J. Nanobiotechnology 2020, 18, 45.
- Xiao, J.; Jiang, X.; Zhou, Y.; Sumayyah, G.; Zhou, L.; Tu, B.; Qin, Q.; Qiu, J.; Qin, X.; Zou, Z.; et al. Results of a 30-day safety assessment in young mice orally exposed to polystyrene nanoparticles. Environ. Pollut. 2022, 292, 118184.
- Chi, Q.; Xu, T.; He, Y.; Li, Z.; Tang, X.; Fan, X.; Li, S. Polystyrene nanoparticle exposure supports ROS-NLRP3 axis-dependent DNA-NET to promote liver inflammation. J. Hazard. Mater. 2022, 439, 129502.
- Zhong, G.; Rao, G.; Tang, L.; Wu, S.; Tang, Z.; Huang, R.; Ruan, Z.; Hu, L. Combined effect of arsenic and polystyrene-nanoplastics at environmentally relevant concentrations in mice liver: Activation of apoptosis, pyroptosis and excessive autophagy. Chemosphere 2022, 300, 134566.
- Liao, H.; Liu, S.; Junaid, M.; Gao, D.; Ai, W.; Chen, G.; Wang, J. Di-(2-ethylhexyl) phthalate exacerbated the toxicity of polystyrene nanoplastics through histological damage and intestinal microbiota dysbiosis in freshwater Micropterus salmoides. Water Res. 2022, 219, 118608.
- Dimitriadis, G.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-Dependent and Insulin-Independent Mechanisms: An Integrative Approach. Nutrients 2021, 13, 159.
- Gregory, J.M.; Kraft, G.; Scott, M.F.; Neal, D.W.; Farmer, B.; Smith, M.S.; Hastings, J.R.; Madsen, P.; Kjeldsen, T.B.; Hostrup, S.; et al. Peripherally delivered hepatopreferential insulin analog insulin-406 mimics the hypoglycaemia-sparing effect of portal vein human insulin infusion in dogs. Diabetes Obes. Metab. 2019, 21, 2294–2304.
- Williamson, G. Effects of Polyphenols on Glucose-Induced Metabolic Changes in Healthy Human Subjects and on Glucose Transporters. Mol. Nutr. Food Res. 2022, 66, 2101113.
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 1273–1298.
- Lee, J.H.; Park, A.; Oh, K.-J.; Kim, W.K.; Bae, K.-H. The Role of Adipose Tissue Mitochondria: Regulation of Mitochondrial Function for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4924.
- Sullivan, M.A.; Forbes, J.M. Glucose and glycogen in the diabetic kidney: Heroes or villains? Ebiomedicine 2019, 47, 590–597.
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Non-alcoholic fatty liver disease: An overview of risk factors, pathophysiological mechanisms, diagnostic procedures, and therapeutic interventions. Life Sci. 2021, 271, 119220.
- Tas, E.; Bai, S.; Mak, D.; Diaz, E.C.; Dranoff, J.A. Obesity, but not glycemic control, predicts liver steatosis in children with type 1 diabetes. J. Diabetes Its Complicat. 2022, 36, 108341.
- Scapaticci, S.; D’Adamo, E.; Mohn, A.; Chiarelli, F.; Giannini, C. Non-Alcoholic Fatty Liver Disease in Obese Youth with Insulin Resistance and Type 2 Diabetes. Front. Endocrinol. 2021, 12, 639548.
- Kosmalski, M.; Ziółkowska, S.; Czarny, P.; Szemraj, J.; Pietras, T. The Coexistence of Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. J. Clin. Med. 2022, 11, 1375.
- Meneses, M.J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M.P. Paraoxonase-1 as a Regulator of Glucose and Lipid Homeostasis: Impact on the Onset and Progression of Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4049.
- Guerra, S.; Gastaldelli, A. The role of the liver in the modulation of glucose and insulin in non alcoholic fatty liver disease and type 2 diabetes. Curr. Opin. Pharmacol. 2020, 55, 165–174.
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216.
- Zhang, X.; He, Z.; Si, Q.; Hu, X.; Yang, L.; Gu, X.; Du, L.; Wang, L.; Pan, L.; Li, Y.; et al. The Association of Sarcopenia and Visceral Obesity with Lean Nonalcoholic Fatty Liver Disease in Chinese Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2022, 2022, 2229139.
- Kwon, O. Glucose Metabolism. In Stroke Revisited: Diabetes in Stroke; Springer: Singapore, 2021; pp. 3–13.
- Bray, G.A.; Bouchard, C. The biology of human overfeeding: A systematic review. Obes. Rev. 2020, 21, e13040.
- Cline, G.W.; Naganawa, M.; Chen, L.; Chidsey, K.; Carvajal-Gonzalez, S.; Pawlak, S.; Rossulek, M.; Zhang, Y.; Bini, J.; McCarthy, T.J.; et al. Decreased VMAT2 in the pancreas of humans with type 2 diabetes mellitus measured in vivo by PET imaging. Diabetologia 2018, 61, 2598–2607.
- Asare-Bediako, I.; Paszkiewicz, R.L.; Kim, S.P.; Woolcott, O.O.; Kolka, C.M.; Burch, M.A.; Kabir, M.; Bergman, R.N. Variability of Directly Measured First-Pass Hepatic Insulin Extraction and Its Association With Insulin Sensitivity and Plasma Insulin. Diabetes 2018, 67, 1495–1503.
- Albrechtsen, N.J.W. The glucose-mobilizing effect of glucagon at fasting is mediated by cyclic AMP. Am. J. Physiol. Metab. 2021, 321, E571–E574.
- Clayton, R.P.; Herndon, D.N.; Abate, N.; Porter, C. The Effect of Burn Trauma on Lipid and Glucose Metabolism: Implications for Insulin Sensitivity. J. Burn Care Res. 2018, 39, 713–723.
- Axsom, J.E.; Schmidt, H.D.; Matura, L.A.; Libonati, J.R. The Influence of Epigenetic Modifications on Metabolic Changes in White Adipose Tissue and Liver and Their Potential Impact in Exercise. Front. Physiol. 2021, 12, 686270.
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223.
- Lim, X.Y. Characterisation of the First Specific Inhibitor of Ceramide Synthase 1. Ph.D. Doctoral Dissertation, University of New South Wales, Sydney, Australia, 2018.
- Kheder, M.H. The Role of the Equine Enteroinsular Axis in Insulin Dysregulation: In Vitro Mechanistic Insights for Disease Prevention. Doctoral Dissertation, Queensland University of Technology, Brisbane City, Australia, 2018.
- Tenen, D.G.; Chai, L.; Tan, J.L. Metabolic alterations and vulnerabilities in hepatocellular carcinoma. Gastroenterol. Rep. 2021, 9, 1–13.
- Yang, X.; Liu, Q.; Li, Y.; Tang, Q.; Wu, T.; Chen, L.; Pu, S.; Zhao, Y.; Zhang, G.; Huang, C.; et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte 2020, 9, 484–494.
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356.
- Lee, J.; Park, J.-S.; Roh, Y.S. Molecular insights into the role of mitochondria in non-alcoholic fatty liver disease. Arch. Pharmacal Res. 2019, 42, 935–946.
- Tharyan, R.G. Transcription Factor nfyb-1 Regulates Mitochondrial Function and Promotes Longevity Induced by Mitochondrial Impairment. Doctoral Dissertation, Universität zu Köln, Köln, Germany, 2019.
- Katayama, K. Zinc and protein metabolism in chronic liver diseases. Nutr. Res. 2020, 74, 1–9.
- Sonti, S.; Tyagi, K.; Pande, A.; Daniel, R.; Sharma, A.L.; Tyagi, M. Crossroads of Drug Abuse and HIV Infection: Neurotoxicity and CNS Reservoir. Vaccines 2022, 10, 202.
- Di Ciaula, A.; Bonfrate, L.; Baj, J.; Khalil, M.; Garruti, G.; Stellaard, F.; Wang, H.H.; Wang, D.Q.-H.; Portincasa, P. Recent Advances in the Digestive, Metabolic and Therapeutic Effects of Farnesoid X Receptor and Fibroblast Growth Factor 19: From Cholesterol to Bile Acid Signaling. Nutrients 2022, 14, 4950.
- Holeček, M. The Role of Skeletal Muscle in The Pathogenesis of Altered Concentrations of Branched-Chain Amino Acids (Valine, Leucine, and Isoleucine) in Liver Cirrhosis, Diabetes, and Other Diseases. Physiol. Res. 2021, 70, 293–305.
- Warrillow, S.; Fisher, C.; Bellomo, R. Correction and control of hyperammonemia in acute liver failure: The impact of continuous renal replacement timing, intensity, and duration. Crit. Care Med. 2020, 48, 218–224.
- Sun, S.-X.; Wu, J.-L.; Lv, H.-B.; Zhang, H.-Y.; Zhang, J.; Limbu, S.M.; Qiao, F.; Chen, L.-Q.; Yang, Y.; Zhang, M.-L.; et al. Environmental estrogen exposure converts lipid metabolism in male fish to a female pattern mediated by AMPK and mTOR signaling pathways. J. Hazard. Mater. 2020, 394, 122537.
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060.
- Wang, Q.; He, G.; Mai, K. Modulation of lipid metabolism, immune parameters, and hepatic transferrin expression in juvenile turbot (Scophthalmus maximus L.) by increasing dietary linseed oil levels. Aquaculture 2016, 464, 489–496.
- Yin, L.; Liu, H.; Cui, H.; Chen, B.; Li, L.; Wu, F. Impacts of polystyrene microplastics on the behavior and metabolism in a marine demersal teleost, black rockfish (Sebastes schlegelii). J. Hazard. Mater. 2019, 380, 120861.
- Pedersen, A.F.; Meyer, D.N.; Petriv, A.-M.V.; Soto, A.L.; Shields, J.N.; Akemann, C.; Baker, B.B.; Tsou, W.-L.; Zhang, Y.; Baker, T.R. Nanoplastics impact the zebrafish (Danio rerio) transcriptome: Associated developmental and neurobehavioral consequences. Environ. Pollut. 2020, 266, 115090.
- Cedervall, T.; Hansson, L.A.; Lard, M.; Frohm, B.; Linse, S. Food Chain Transport of Nanoparticles Affects Behaviour and Fat Metabolism in Fish. PLoS ONE 2012, 7, e32254.
- Yan, J.; Liao, K.; Wang, T.; Mai, K.; Xu, W.; Ai, Q. Dietary Lipid Levels Influence Lipid Deposition in the Liver of Large Yellow Croaker (Larimichthys crocea) by Regulating Lipoprotein Receptors, Fatty Acid Uptake and Triacylglycerol Synthesis and Catabolism at the Transcriptional Level. PLoS ONE 2015, 10, e0129937.
- Lai, W.; Xu, D.; Li, J.; Wang, Z.; Ding, Y.; Wang, X.; Li, X.; Xu, N.; Mai, K.; Ai, Q. Dietary polystyrene nanoplastics exposure alters liver lipid metabolism and muscle nutritional quality in carnivorous marine fish large yellow croaker (Larimichthys crocea). J. Hazard. Mater. 2021, 419, 126454.
- Huang, T.; Zhang, W.; Lin, T.; Liu, S.; Sun, Z.; Liu, F.; Yuan, Y.; Xiang, X.; Kuang, H.; Yang, B.; et al. Maternal exposure to polystyrene nanoplastics during gestation and lactation induces hepatic and testicular toxicity in male mouse offspring. Food Chem. Toxicol. 2022, 160, 112803.
- Shi, C.; Han, X.; Guo, W.; Wu, Q.; Yang, X.; Wang, Y.; Tang, G.; Wang, S.; Wang, Z.; Liu, Y.; et al. Disturbed Gut-Liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environ. Int. 2022, 164, 107273.
- Yin, K.; Wang, D.; Zhang, Y.; Lu, H.; Wang, Y.; Xing, M. Dose-effect of polystyrene microplastics on digestive toxicity in chickens (Gallus gallus): Multi-omics reveals critical role of gut-liver axis. J. Adv. Res. 2022; (In Press).
