Intravenous immune globulin (IVIG) is made after processing plasma from healthy donors. It is composed mainly of pooled immunoglobulin and has clinical evidence-based applications in adult and pediatric populations.
- immunoglobulins
- fetus
- neonates
- sepsis
- hemolysis
- hyperbilirubinemia
- necrotizing enterocolitis
1. Introduction
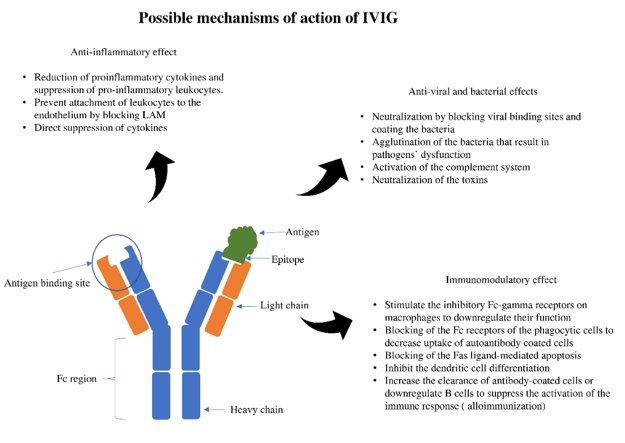
Immunoglobulin therapy is defined as the use of a combination of antibodies obtained from healthy human donors to treat different conditions [1][2][1,2]. The principal components of intravenous immunoglobulin (IVIG) are IgG antibodies, which compromise about 90% of the IVIG. Antibodies are glycoproteins synthesized and secreted by plasma cells (activated B cells) to respond to antigenic stimulation with the primary purpose of a specific immune response to result in different physiological and/or pathological processes [2][3][2,3]. The basic structural unit is primarily formed by two heavy and two light chains [4][5][4,5]. The difference between the heavy chains results in different kinds of antibodies: IgG, IgA, IgM, IgE, and IgD. After synthesis, formed antibodies functions by binding with a specific antigen epitope. This binding subsequently results in specific actions that ultimately help neutralize and inactivate the pathogenic organisms or trigger a specific immune response (Figure 1).
Figure 1. Antigen-antibody binding and specific effects. LAM; leukocyte adhesion molecule.
2. Clinical Use in Fetuses and Neonates
2.1. Alloimmune Hemolytic Disease in Neonates
Alloimmune hemolytic disease (AIHD) of the newborn, otherwise known as the newborn's hemolytic disease, is considered the most common cause of hemolytic disease in the neonatal period [6][7][8][9][27–30]. AIHD is regarded as the first and the most common indication for IVIG to prevent severe hyperbilirubinemia that may require an exchange transfusion [10][11][31,32]. This condition's primary pathophysiology is due to the hemolysis of neonatal red blood cells by maternally-derived IgG antibodies [12][33]. These antibodies are derived in the maternal blood during pregnancy or shortly after when the incompatible fetal antigen enters the maternal circulation. Once these antibodies are produced, there is a potential for transplacental transfer from the maternal circulation to the fetal blood; this transfer leads to the possibility of antigen-antibody interaction that ultimately may result in hemolysis [13][34]. The primary two types of AIHD are ABO (The major human blood group system) incompatibility and RhD (Rhesus factor D) hemolytic disease. In the ABO form, hemolysis occurs due to existing antibodies. For example, mothers with A and B blood groups only produce IgM, which does not cross the placenta, and isoimmunization does not occur. In type O mothers, the antibodies are predominantly IgG, which crosses the placenta and can cause hemolysis in the fetus. Unlike Rh, ABO disease can occur in first pregnancies because anti-A and anti-B antibodies are found early in life from exposure to A- or B-like antigens present in many food items and other natural substances. Further, hemolysis is less severe in ABO because A and B antigen is expressed by other cell types such as endothelial cells, thus diluting the effect of circulating antibodies. On the other hand, RhD hemolytic disease usually happens during birth or pregnancy. When the mother is exposed to the paternally derived RhD antigen for the first time, IgM antibodies are formed. However, due to their relatively large size, IgM antibodies do not cross the placental barrier. Therefore first pregnancies are usually not affected. During subsequent pregnancies and repeated exposure, IgG antibodies are formed, and they can potentially transfer via the placenta and attack fetal red blood cells and ultimately result in their breakdown [14][35]. RhD hemolytic disease used to be the most common cause of severe alloimmune hemolysis in neonates. However, the emergence of antenatal Rh (D) prophylaxis and the intrauterine intervention offered to the affected fetuses resulted in a significant decrease in the occurrence and the severity of hemolysis associated with Rh (D) hemolytic disease [15][16][36,37]. Due to this decrease in incidence, hemolysis due to ABO incompatibility became more common; however, only about 15% of the affected pregnancies with ABO incompatibility will develop hemolysis. Only a smaller percentage will develop severe hyperbilirubinemia [17][18][38,39]. In the presence of hemolysis unexplained by either RhD or ABO incompatibility, investigation for other minor blood groups (Duffy, Kell, P, and others) or different Rh antigens (E, C, and c) incompatibility is recommended.
IVIG has been proposed as a potential intervention that can decrease hemolysis severity and, therefore, the associated hyperbilirubinemia [19][20][47,48]. The exact mechanism of the action of IVIG to reduce hemolysis is unclear. Scientists suggest IVIG works most likely by blocking the antibodies' receptors located on the red blood cells' surface. Blocking these receptors will prevent the antigen/antibody interactions between the antigens found on the red blood cells and the maternal antibodies, decreasing recognition of the targeted red blood cells by the circulating macrophage and subsequently decreasing the degree of hemolysis [21][32,49]. The first reported use of IVIG in AIHD of the newborn was published in 1987 [22][20]. This report was followed by other case reports and case series that suggested using IVIG as a useful intervention to halt severe hyperbilirubinemia [23][24][25][50–52]. This intervention's primary beneficial effect is to decrease the need for exchange transfusion (a high-risk procedure performed in advanced intensive care units to prevent the risk of bilirubin-induced brain damage).
2.2. Neonatal and Fetal Alloimmune Thrombocytopenia
Fetal and neonatal alloimmune thrombocytopenia (FNAIT) is thrombocytopenia caused by maternal-fetal antiplatelet antibodies, resulting in platelet destruction.[26][27][62,63]. The most commonly accepted theory for pathophysiology is maternal IgG antibody formation against fetal paternally derived antigens. These IgG antibodies can pass through the placenta and subsequently form an antigen-antibody complex that ultimately results in platelet destruction.
Many platelet antigens have been identified as potential triggers for this immune process. Human platelet antigen (HPA)-1a is considered the most common trigger for maternal antibody formation and hence fetal and neonatal thrombocytopenia [28][64]. FNAIT usually affects the first-born child more than subsequent children, in contrast to AIHD, which affects subsequent pregnancies with a more severe degree of hemolysis [27][63]. Postnatal clinical manifestations vary significantly. More than half of the cases are asymptomatic and identified mainly by screening complete blood count laboratory evaluation for other reasons. Severe thrombocytopenia can lead to rapid progressive bleeding. The most feared complication is spontaneous intracranial hemorrhage (ICH) [29][30][65,66].
The main principles of treatment of FNAIT are anticipation, antenatal IVIG therapy, postnatal recognition, and timely interventions. Risk anticipation is based on the maternal history of a previous child who developed thrombocytopenia during the second or the last third of gestation or shortly after delivery due to FNAIT in previous pregnancies. The mothers identified have a risk for subsequent deliveries with potential risk for ICH,
Multiple studies have addressed the benefits of antenatal management of FNAIT. Bussel et al. found in their prospective study that IVIG administration in mothers who had a history of infants affected by FNAIT resulted in a significant increase in fetal platelet counts. None of the neonates had ICH [31][67]. A recent meta-analysis was done by Winkelhorst et al. to evaluate the effect of IVIG in the management of FNAIT. Four randomized trials and 22 nonrandomized observations were included in the analysis; however, pooling for statistical analysis was not feasible due to the significant heterogenicity. The study found that IVIG treatment with or without the addition of corticosteroids at different dosing regimens is a reasonable approach when considering antenatal management to prevent the risk of bleeding in the affected neonates [30][66]. Risk stratification and suggested dosing regimens are shown in Table 2 [32][66,68]. The justifications for aggressive management once affected mothers are identified are the high rate of recurrence in subsequent pregnancies and the need to prevent ICH's devastating outcome.
2.3. Neonatal Hemochromatosis
Neonatal hemochromatosis (N.H.), also known as gestational alloimmune liver disease (GALD), is a rare condition that affects neonates. The pathophysiology is best explained as an immune process caused by the maternal transfer of IgG antibodies directed toward antigens located on the fetal hepatocytes [33][114]. The prognosis was poor for this condition, as most infants affected developed severe liver failure [34][115]. Traditional treatment for N.H. depended mostly on the use of chelating agents to decrease iron disposition in the hepatocytes. However, this treatment showed only mild improvement. Most of the treated infants required liver transplantation to improve survival. With a greater in-depth understanding of this condition's pathophysiology, newer treatment modalities have focused on controlling the immune-mediated process [35][116].
Treatment protocols were developed to prevent the severe consequences of anticipated N.H. by intervening during pregnancy. Whittington et al. showed in a relatively large sample that IVIG administered during pregnancy to mothers who had previous infants who developed N.H. resulted in significantly better outcomes. A total of 188 pregnant women who received treatment were compared to other women with high-risk pregnancies who did not receive treatment. The final analysis showed a significant difference in outcomes, as only 30% of the untreated pregnancies resulted in healthy infants compared to 94% in the treated group [36][80].
Another recent report, by Okada, et al., noted a significantly favorable outcome for infants with neonatal hemochromatosis by administering IVIG to high-risk N.H. pregnancies. In their small trial, they treated a total of eight pregnancies in six women with IVIG (1 g/kg) administered at the beginning of the second trimester at weekly or biweekly dosing frequency. This regimen was continued until 18 weeks of gestation, then weekly until the time of delivery. Only three out of the eight infants born in this study developed liver dysfunction. In these three infants, the abnormalities were transient and resolved without treatment [37][117].
Another successful approach used a double exchange transfusion followed by IVIG (1g/kg) immediate administration to clear the attacking maternal antibodies. Rand et al. reported promising outcomes and significantly improved prognosis defined as survival without the need for a liver transplant. In total, 12 out of 16 (75%) infants with N.H. survived without the need for liver transplantation compared to only 23 (17%) out of 131 infants in the historical control group [38][81]. Further reports confirmed favorable outcomes with a similar postnatal management approach [39][40][118,119].
2.4. Primary Immunodeficiency
Making the diagnosis of primary immunodeficiency in neonates may be challenging in the first few months of life due to the presence of maternal antibodies in the fetal circulation [41][120]. However, certain conditions may manifest early in life, mainly those associated with a severe deficiency in the humoral immune response (X-linked hyper-IgM syndrome, severe combined immunodeficiency (SCID), X-linked agammaglobulinemia, and others). Treatment with IVIG in this age group remains controversial. IVIG replacement therapy is primarily indicated for those with recurrent severe or unusual infections associated with different types of immune deficiencies like hypogammaglobulinemia, common variable immune deficiency, and others.
Agammaglobulinemia, due to the complete absence of B cells, requires IVIG replacement therapy to protect against different pathogens in this critical period of life [22]. The dosing regimen and administration schedule can vary, but generally, achieving an IgG level goal of 500 mg/dl to 800 mg/dl is recommended to prevent serious complications. Usually, these levels are achieved with lifelong administration of IVIG of 400–500 mg/kg at monthly intervals [42][82,121].
2.5. Kawasaki Disease
Kawasaki disease (K.D.) is a form of vasculitis that affects the pediatric population. The pathophysiology for this condition is not clearly known. Multiple hypotheses and theories have been proposed to explain the disease process, with infectious or autoimmune triggering or both being the most widely accepted explanation [43][122]. K.D. disease rarely affects neonates. Only a few cases have been reported in the literature [44][45][123,124]. The low incidence in the neonatal group is possibly related to the immature immune system and the relatively high concentration of maternal antibodies in the neonatal circulation.
The treatment algorithm in neonates affected with K.D. follows the same steps involved in the pediatric population [46][83]. Although high-dose aspirin and IVIG (2 g/kg) usually result in complete recovery in the majority of pediatric cases, the response is less predictable in the neonatal cases [45][47][124,125].
2.6. Neonatal Lupus
Neonatal lupus is the description used to describe infants who are affected by maternally derived antibodies (anti-Ro/SSA and anti-La/SSB). These antibodies can be found in the maternal serum with multiple autoimmune conditions, not limited to systemic lupus erythematosus.
Congenital atrioventricular heart block (AVHB) associated with neonatal lupus carries a significant risk for morbidity and mortality. The rule of IVIG use in this condition has been studied at a different dosing regimen with and without other interventions (dexamethasone and plasmapheresis). Friedman et al. showed in their prospective open-label trial that the use of low dose IVIG at 400 mg/kg in pregnant mothers who had a previous newborn with AVHB did not prevent the recurrence in subsequent pregnancies [48][126]. Other small-scale trials using a combination management approach consisting of weekly plasmapheresis, IVIG every two weeks and continued soon after birth, in addition to betamethasone, have shown to be safe and effective in second-degree heart block but not in complete heart block [49][127].
In thrombocytopenia due to maternally derived antibodies seen in infants born to mothers with an autoimmune condition, IVIG use at 1 g/kg/day for two days or 0.5 g/kg/day for four days may improve affected neonates platelets counts [50][73].