To achieve sustainable development, alternative resources should replace conventional resources such as fossil fuels. In marine ecosystems, many macroalgae grow faster than terrestrial plants. Macroalgae are roughly classified as green, red, or brown algae based on their photosynthetic pigments. Brown algae are considered to be a source of physiologically active substances such as polyphenols. Furthermore, some macroalgae can capture approximately 10 times more carbon dioxide from the atmosphere than terrestrial plants. Therefore, they have immense potential for use in the environment. Macroalgae have emerged as a biomass feedstock for bioethanol production owing to their low lignin content and applicability to biorefinery processes.
- macroalgae
- phlorotannin
- molecular display
- bioethanol
- xylan
- mannitol
- laminarin
- alginate
1. Introduction
2. Biological Activity and Bioconversion of Green Macroalgae
2.1. Ulvan
The green macroalgae Ulva species are edible seaweeds comprising health-promoting and bioactive compounds. The major carbohydrates of Ulva species are ulvans and glucans, with median values of 45.0 mol% and 22.5 mol% for rhamnose and glucuronic acid, respectively. Ulvan accounts for 9–36% of the dry weight of Ulva species [12][21]. It is high in dietary fiber, thereby promoting gastrointestinal health, and is associated with a decrease in the occurrence of chronic diseases.2.2. L-Rhamnose
Rhamnose is an important monosaccharide that is widely distributed among microorganisms and plants. Certain bacterial saponin glycans contain rhamnolipids, mycolic acids, and extracellular polysaccharides [13][25]. In the green macroalga Ulva lactuca, L-rhamnose and D-glucose are the major carbohydrates present in the ulvan polysaccharide structure; these sugars can be recovered under mild conditions [14][26]. Investigation of the antiviral activity of rhamnose polysaccharides revealed that rhamnose sulfate in the green alga Monostroma nitidum exhibits anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) activity. SARS-CoV-2 invasion is achieved via the interaction of its S-protein with angiotensin-converting enzyme 2 (ACE2) in susceptible host cells. The rhamnose fraction not only inhibited the binding of S-protein and ACE2 analogs but also that of SARS-CoV-2 and ACE2 analogs [15][27]. Clostridium beijerinckii can use L-rhamnose as the sole carbon source to produce acetic acid, butyric acid [16][32], 1,2-propanediol, propionic acid, and n-propanol [17][33]. Green macroalgae can be processed into hydrolysates containing D-glucose and L-rhamnose; therefore, they have potential applications as an industrial fermentation strain. D-galactosyl-β1→4-rhamnose, which exerts immunomodulatory activity, is produced by a one-pot reaction using a combination of recombinant phosphorylases and dried baker’s yeast [18][34].2.3. Bioconversion Using Yeast Cells
As an example of a yeast-based bioconversion application, Greetham et al. investigated the fermentation ability of the marine yeast Wickerhamomyces anomalus M15, particularly for hydrolysis and ethanol production, using brown (Laminaria digitata), green (Ulva linza), and red (Porphyra umbilicalis) macroalgae [19][35]. After pretreatment with seawater, the highest amount of sugar was liberated by the green macroalga U. linza.3. Component and Bioconversion of Red Macroalgae
Carrageenan is the main carbohydrate component in red macroalgae such as Eucheuma denticulatum [20][37], and agar is the main component in species such as Gelidium amansii [21][38]. During the decomposition of agarose, enzymatic hydrolysis, acid hydrolysis, and acid prehydrolysis with subsequent enzymatic hydrolysis result in the liberation of 3,6-anhydro-α-L-galactose (AHG) and D-galactose for subsequent fermentation [22][39]. Many marine microorganisms, including Pseudoalteromonas carrageenovora [23][43], Zobellia galactanivorans [24][44], Pseudoalteromonas fuliginea [25][45], and Saccharophagus degradans [26][46], exhibit agarase activity. Additionally, the catabolic pathway of AHG has been well investigated in the agarolytic marine bacteria Vibrio sp. [27][47] and Streptomyces sp. [28][48]. As a yeast-based application in bioconversion, a study investigated ethanol production using a hydrolysate derived from the red macroalga Gracilaria verrucosa [29][50]. Analysis of the relationship between galactose adaptation effects and mRNA transcriptional levels revealed that the use of galactose for ethanol fermentation using Gracilaria verrucosa hydrolysates enhanced the overall ethanol yield in Saccharomyces cerevisiae KCCM 1129 [29][50].4. Bioactivity of Brown Macroalgae
4.1. Macroalgae Polyphenols or Phlorotannins
Polyphenols are compounds that contain more than two hydroxyl groups. Flavonoids, lignin, and tannins are well-known polyphenols produced by terrestrial organisms [30][52]. Tannins are further categorized into condensed tannins, which are formed by polymerized flavanols, and hydrolyzable tannins, which are combined with sugar and gallic or ellagic acid via ester bonds [31][53]. Polyphenols in macroalgae, known as phlorotannins, have a polymerized structure of phloroglucinol and are different from the tannins in terrestrial organisms.4.2. Inhibiting Advanced Glycation End Product (AGE) Formation
AGEs are produced by nonenzymatic reactions between proteins and reducing sugars [32][33][56,57]. AGEs play important roles in the development of diabetic complications, osteoporosis, atherosclerosis, sarcopenia, and neuropathy [34][35][58,59]. Chemical synthesis has been used to develop glycation inhibitors to suppress AGE production. For example, aminoguanodine [36][60] and OPB-9195 [37][61] have been identified as AGE inhibitors; however, they have not been approved for clinical use owing to their adverse effects. Therefore, compounds that are effective against AGE formation have been explored in edible plants [38][62].4.3. Effect of Phlorotannins on Methylglyoxal (MGO) Formation
AGEs are produced after the formation of MGO, an α-dicarbonyl compound [39][63]. Studies have reported that the blood MGO levels were higher in patients with type I diabetes than in healthy people [40][41][64,65]. Therefore, phlorotannins extracted from Lessoniaceae were evaluated for their inhibitory activities against fluorescent AGE production in human and bovine serum albumin (HSA and BSA)–MGO models [42][66]. The inhibitory effect on the formation of fluorescent AGEs was calculated as the half-maximal inhibitory concentration (IC50).4.4. Effect of Phlorotannins on Glyceraldehyde (GA) Formation
GA is also involved in AGE production. AGEs derived from GA form faster than those from MGO [43][67]. Therefore, many studies have explored inhibitors of the formation of AGEs from GA [44][45][46][68,69,70]. In addition to the serum albumin–MGO models described in the previous section, the inhibitory effects of phlorotannins have been examined using HSA– or BSA–GA models [47][71]. As a result, phlorotannins from Lessoniaceae exhibited an IC50 of 0.48–0.70 mg/mL. The inhibitory effect of phlorotannins derived from Eisenia bicyclis on fluorescent AGEs was 2.3–3.7-fold higher than that of AG as a positive control.4.5. Effect of Phlorotannins on Nε-(Carboxymethyl)lysine (CML)
CML is an AGE formed by the oxidation of glucose with lysine [48][72]. In human dermal fibroblasts, CML–collagen decreased the ability of epidermal keratinocytes to adhere to collagen and induce apoptosis [49][73]. CML–collagen inhibits collagen cross-linking in osteoblasts and causes diabetic osteopenia [50][51][74,75]. The suppression of CML formation in these diseases is thought to be clinically important. Recently, the inhibitory effect of phlorotannins on CML formation was examined [52][76]. The inhibitory effect following treatment with phlorotannins from Lessoniaceae on CML formation was 0.16 μg/mL, which was distinctively lower than that following treatment with 0.40 mM AG as a positive control.5. Microbial Conversion of Macroalgae
5.1. Microorganisms and Their Enzymes
To develop bioconversion methods for algae, an effective method for crushing and saccharifying seaweed bodies is crucial. Considering these situations, algae-degrading microorganisms can be exploited to develop a sustainable tool for algal processing. Previous outbreaks of seaweed diseases have led to the screening of algae-degrading bacteria. The marine bacterium Alteromonas elyakovii KMM 162T was isolated from spot-wounded fronds of the brown macroalga Laminaria japonica [53][77]. Furthermore, genes encoding alginate lyase family PL-7, an oligoalginate lyase classified as alginate lyase (family PL-17), 4-deoxy-l-erythro-5-hexoseulose uronic acid (DEH) reductase, KdgF, 2-keto-3-deoxy-D-gluconate (KDG) kinase, and 2-dehydro-3-deoxy-phosphogluconate aldolase have been identified [54][80]. The KDG produced by this cluster is further metabolized in a major biochemical pathway of sugars. Using this gene cluster, Formosa haliotis may effectively and functionally use fewer compounds in marine environments than in terrestrial environments [54][80].5.2. Degradation of Alginate
Alginate-degrading bacteria are considered industrially important because products using alginate lyases can be applied in the pharmaceutical industry, the food industry, and bioethanol production [55][81]. Several researchers have investigated alginolytic strains in the environment and identified them as Sphingomonas sp. strain A1 [56][82], Zobellia galactanivorans [57][83], Vibrio splendidus strain 12B01 [58][84], and Saccharophagus degradans strain 2–40 [59][85]. Alginate-degrading bacteria have been further explored for the efficient production of rare sugars from brown macroalgae by screening algae-corrupting bacteria. As a result of this screening, Falsirhodobacter sp. strain alg1 was isolated and analyzed [55][60][81,86].5.3. Immobilization of Recombinant Alginate Lyase
To achieve effective and sustainable DEH production, microbial strains of Escherichia coli, Saccharomyces cerevisiae, and Sphingomonas sp. A1 were developed by introducing genes encoding alginate lyase and other enzymes related to DEH fermentation and bioethanol production [61][62][87,88]. These strains can produce ethanol directly from sodium alginate. The enzymatic reactions of recombinant endo-alginate lyase Alg7D and exo-alginate lyase Alg17C from Saccharophagus degradans yielded 45.5% DEH (DEH weight/alginate weight) from alginate [63][89]. Considering the industrial applications of DEH, increasing the yield of DEH and examining the reusability of enzymes are warranted to minimize costs. In general, enzyme reusability can be attained by immobilizing the enzymes into carrier materials. Moreover, the immobilized enzymes can be handled as solids and readily separated from the reaction mixture containing the products. Tanaka et al. examined DEH production using free and immobilized alginate lyases, endo-type AlyFRA, and exo-type AlyFRB from Falsirhodobacter sp. alg1 [64][90].6. Molecular Display Technology for Macroalgae Utilization
6.1. Technology for Immobilizing Proteins on the Cell Surface
After the development of genetic engineering, molecular display technology or cell surface engineering was developed for various biological investigations and was conveniently applied to prepare recombinant proteins in bioprocesses [65][66][67][91,92,93]. The first technology in this field was the so-called “phage display” technology, which was developed by Smith [68][94]. In this technology, a foreign protein is inserted into the filamentous phage protein III via genetic manipulation, and its fusion protein is produced on the virion surface. This technology is currently used for screening combinatorial proteins or clones of peptide ligands [69][70][95,96]; however, it is difficult to perform and involves steps such as infection of Escherichia coli cells with phages for the recovery of positive clones. This technical challenge can be solved using molecular display technology with bacterial cells, which can provide an easier display system without infection and can display large numbers of proteins [71][72][97,98].6.2. Yeast Display System
The yeast Saccharomyces cerevisiae is well known as a useful host of genetic biotechnology because it can fold and glycosylate heterologous eukaryotic proteins. Furthermore, these cells are economically advantageous for high-density cultivation. Moreover, yeast cells can be used to express different proteins using several genetic markers. Indeed, various studies have reported that yeast can display different kinds of protein, the so-called “co-display” [73][74][75][105,106,107]. This molecular display system enabled us to perform high-throughput screening using conventional devices such as a flow cytometer or a multiwell plate reader [76][77][108,109]. The cell surface of the yeast Saccharomyces cerevisiae comprises β-glucans and mannoproteins [78][110], which exist outside the cell membrane. Cell wall proteins, such as agglutinins (Aga1 and Aga2), Flo1, Sed1, and Cwp1, are well-known anchor molecules that can retain target proteins on the yeast cell surface. In addition to these proteins, α-agglutinin is also one of the most widely used anchoring proteins for heterologous proteins in the yeast display system. A target and α-agglutinin fusion protein is produced by introducing multicopy plasmids or integrative plasmids into the host strain. A fusion protein in the system is transiently transported to the exterior of the cell membrane by secretory vesicles and then released by an enzymatic reaction involving phosphatidylinositol-specific phospholipase C. Finally, the target–α-agglutinin fusion protein is transferred to the cell wall [79][80][111,112].6.3. Bioethanol Production from Laminarin
As described earlier, brown algae have the potential to be used to produce biomass energy because they do not compete with food and do not contain persistent lignin. As mentioned in the Introduction, brown algae contain up to 35% lignin on a dry weight basis [81][16] and have attracted much attention in the field of energy production. Nevertheless, they have not yet been effectively used as biomass because they cannot decompose into glucose. Therefore, to use brown algae, it is necessary to degrade laminarin to produce glucose for assimilation during alcohol fermentation. Laminarinase, i.e., β-1,3-glucanase and β-1,6-glucanase, can produce glucose from polysaccharides for ethanol production [82][117]. Studies have reported ethanol production from laminarin using microorganisms [83][84][85][118,119,120]. Pichia angophorae can directly produce ethanol from laminarin [85][120]; however, it does not exhibit salt tolerance, unlike Saccharomyces cerevisiae [86][121]. In bioethanol production from brown macroalgae, the salt-tolerant characteristics of microbial cells would be advantageous. Although Gly16G and Lam16B have already been predicted to be laminarinases [87][123], the catalytic machinery of Gly16G is missing according to NCBI (http://www.ncbi.nlm.nih.gov/ accessed on 1 March 2023). Moreover, the molecular weight of Lam16B is extremely high and is therefore thought to be unsuitable for cell surface displays [88][89][124,125]. As a result, Gly5M was selected as the candidate hydrolytic enzyme for laminarin and displayed on the yeast cell surface. In the reaction between laminarin and Gly5M-displying yeast, oligosaccharides were produced, and Gly5M was suggested to be a novel hydrolytic enzyme for laminarin. Analysis of the produced oligosaccharides revealed that most comprised gentiobiose, with two glucose molecules linked by a β-1,6-glycosidic bond.6.4. Bioethanol Production from Xylan
Xylan is present in macroalgae and comprises a heteropolysaccharide with β-1,4-linked xylopyranoside. It constitutes >90% of the hemicellulose content [90][126]. Bioconversion of xylan into bioethanol can be an efficient and sustainable method for bioethanol production from nonedible biomass derived from macroalgae. In a previous study, xylan-degrading xylanase II (XYNII) from Trichoderma reesei and beta-xylosidase (XylA) from Aspergillus oryzae were codisplayed [91][127]. The XYNII- and XylA-displaying strain was used for direct ethanol production from birchwood xylan. The strain could produce D-xylose using the displayed enzymes, and fermentation of D-xylose was achieved by introducing the oxidoreductase-based enzymes NAD(P)H-dependent D-xylose reductase and xylitol dehydrogenase [92][128]. Another route for the production of xylose involving isomerase (XI), which is predominantly derived from bacteria and catalyzes the isomerization of D-xylose into D-xylulose, has been investigated [93][131]. XI does not require coenzymes for isomerization. Moreover, using XI, higher theoretical yields (0.51 g ethanol/g xylose) can be achieved compared with the conventional pathway (0.46 g ethanol/g xylose) [94][132].6.5. Bioethanol Production from Alginate and Mannitol
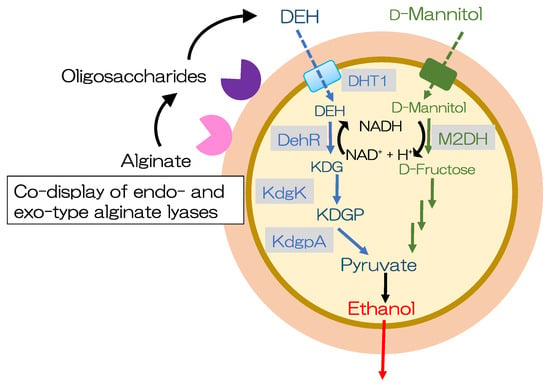
Introduction of the DEH transporter and components of the DEH metabolic pathway (DehR, KdgK, and KdgpA) into Saccharomyces cerevisiae is required for DEH assimilation because Saccharomyces cerevisiae cannot assimilate DEH. Enquist-Newmam et al. constructed a Saccharomyces cerevisiae strain that can use both DEH and mannitol [95][141]. To screen for a DEH transporter, the Saccharomyces cerevisiae strain BAL2193 was constructed by genomically integrating genes for DEH assimilation (dehR from Sphingomonas sp. strain A1, kdgK from Saccharophagus degradans, and kdgpA from Vibrio splendidus). Codon-optimized dehR from Vibrio harveyi, kdgK from Escherichia coli, and kdgpA from Vibrio splendidus were selected for engineering Saccharomyces cerevisiae using an enzymatic assay of the cell lysate and ethanol productivity. The resulting strain produced 36 g/L ethanol from a 98 g/L sugar mixture (alginate and mannitol). The metabolically modified Saccharomyces cerevisiae could generate ethanol from DEH and mannitol; however, unmodified Saccharomyces cerevisiae lacked the ability to utilize alginate [95][141]. Yeast molecular display technology has been further improved for direct ethanol production from alginate and mannitol in brown macroalgae (Figure 13) [96][143]. First, the genes encoding the components of the DEH pathway that produce ethanol directly from alginate and mannitol were examined. Then, the genes encoding Alg7A and Alg7K from Saccharophagus degradans, DHT1 from Asteromyces cruciatus, dehR from Vibrio splendidus, and kdgK from Escherichia coli were examined. Furthermore, mannitol-metabolizing capacity was enhanced to control the redox balance during prolonged cultivation using a medium with mannitol as the sole carbon source. The resulting strain, alginate- and mannitol-assimilating (AM1), was cultivated in a medium containing 6% (w/v) of total sugar (approximately 1:2 ratio of alginate/mannitol). The strain could directly produce ethanol from alginate and mannitol and obtained 8.8 g/L of ethanol, with yields of up to 32% of the theoretical yield [96][143].