Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Landry Rali Hakiza and Version 2 by Jessie Wu.
Obesity is a chronic relapsing disease of global pandemic proportions. In this context, an increasing number of patients are undergoing bariatric surgery, which is considered the most effective weight loss treatment for long-term improvement in obesity-related comorbidities. One of the most popular bariatric surgeries is the Roux-en-Y gastric bypass (RYGB).
- endoscopic transoral outlet reduction
- bariatric endoscopy
- obesity
- gastric bypass
- dumping syndrome
- weight
1. Transoral Outlet ReductionRe for Weight Regain after Roux-en-Y Gastric YGBypass
Weight recidivism is a common complication following Roux-en-Y gastric bypass (RYGB)GB surgery. On average, patients regain between 20 and 30 % of lost weight, and moreover, excessive weight gain is experienced by over one third of patients [1][2][11,12]. Weight regain after gastric bypass is often multifactorial and can be attributed to eating patterns, and psychological and social factors. However, dilatation or enlargement of the gastrojejunal anastomosis of >30 mm is a significant predictor of weight regain following RYGB [3][4][5][13,14,15]. Due to the technical complexity of the anatomy, surgical re-intervention is accompanied by a high risk of complications and an increase in postoperative morbidity and mortality [6][16]. As an alternative, transoral outlet reduction (TORe) was developed in 2013 as an endoscopic procedure focusing on reducing the size of the gastrojejunal anastomosis (GJA) [7][17]. The first interventional study included 25 patients with an average weight regain of 24 kg after RYGB [7][17]. This research tudy described endoscopically reducing the diameter of the anastomosis by an average of 77.3% which was associated with an average weight loss of 11.5 kg, 11.7 kg and 10.8 kg at 3, 6 and 12 months, respectively [7][17].
Vargas et al. demonstrated in a multicenter study that TORe is a safe, reproducible and effective approach to managing weight recidivism after RYGB [8][18]. The average weight loss at 6, 12 and 18 months was 9.31 ± 6.7 kg, 7.75 ± 8.4 kg and 8 ± 8.8 kg, respectively, and no serious adverse events were reported [8][18].
2. Transoral Outlet ReductionRe for Dumping Syndrome after Roux-en-Y Gastric YGBypass
Dumping syndrome (DS) is a postprandial phenomenon in which patients present with a constellation of gastrointestinal and vasomotor symptoms, including tachycardia, fatigue, syncope, and occasionally, shock and seizures due to profound hypoglycemia [9][20]. Symptoms may occur early (within 1 h of a meal) or up to 3 h later, the latter being associated with postprandial hypoglycemia. As the name suggests, DS occurs, in part, due to rapid gastric emptying, leading to rapid passage of food into the small intestine [10][11][21,22]. The patient’s typical history and blood sugar determination inform the diagnosis. The Sigstad score (a score >7 is strongly suggestive of dumping) and questionnaires may also be helpful [10][21].
A conservative stepwise approach is currently recommended, starting with dietary changes in the form of more frequent meals with increased protein content and lower overall carbohydrate content, favoring complex carbohydrates [12][13][23,24]. If dietary measures prove unsuccessful, drug therapy can be initiated with acarbose, calcium antagonists or GLP-1 analogues [14][25].
However, dietary restrictions and pharmacological treatments are often ineffective or poorly tolerated [10][11][21,22]. In these cases, TORe provides a solution by reducing the speed of gastric emptying, however there is no clear consensus in the literature regarding the place of surgical re-intervention in treating dumping syndrome [10][15][16][17][21,26,27,28].
A large study involving 115 patients from two large academic centers in the United States and Germany supported TORe as an effective and safe adjuvant therapy to lifestyle and pharmacologic treatment of refractory DS [18][29]. The Sigstad score reduced significantly after only 3 months post-TORe, with the mean sore changing from 17 ± 6.1 to 2.6 ± 1.9 [18][29]. Similarly, Brown et al. demonstrated a 90% rate of resolution of DS after only 3 months of revision [19][30].
3. Transoral Outlet ReductionRe Technique
TORe is currently the most frequently used technique for the reduction of a dilated GJA (Figure 1A). The intervention is usually performed under general anesthesia. A double-lumen gastroscope is passed through a proprietary overtube of 25 cm in length, and CO2 is used for insufflation. It can be carried out on an outpatient basis, and it is typically performed with argon plasma coagulation (APC) combined with full-thickness suturing achieved using the OverStitchTM device (Apollo Endosurgery, Austin, TX, USA) [5][20][21][15,32,33]. This combined technique allows for greater durability of anastomotic reduction by inducing fibrosis of the GJA [22][23][34,35]. The first step of the procedure is to ablate the gastric rim of the anastomosis via APC (forced APC, 0.8 L/min with 30–70 watts) (Figure 1B), followed by a circumferential, transmural endoscopic suture (Figure 1C). Suturing is mainly performed via the creation of a purse-string, or alternatively, by placing interrupted sutures at the GJA [18][29]. The purse-string technique is, however, generally favored, as it results in more significant weight loss at one year than interrupted suture patterns [24][36]. Ideally, a dilation balloon (CRE balloon dilator, Boston Scientific, Marlborough, MA, USA) is introduced through the second channel of the endoscope and inflated to a diameter of 8–10 mm (Figure 1D) to size the GJA before the suture is tightened and cinched over the balloon, allowing the GJA to be precisely sized (Figure 1E and Video S1).
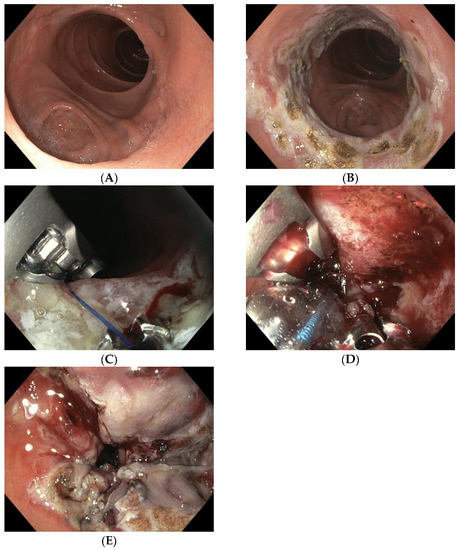
Figure 1. (A) Dilated GJA; (B) ablation of the gastric rim via APC; (C) suturing the anastomosis with the Apollo Overstitch system; (D) suture size control using an 8 mm CRE balloon; (E) narrowed GJA after TORe.
There are several other TORe techniques described in the literature [23][25][26][27][28][35,37,38,39,40]. Initially, some studies demonstrated efficacy using APC alone in the GJA, which was relatively simple to perform, and even feasible with patients under conscious sedation [29][30][31][32][41,42,43,44]. Jaruvongvanich et al. reported a meta-analysis showing that both full-thickness suturing plus APC (ft-TORe) and argon plasma mucosal coagulation alone (APMC-TORe) offer comparable weight loss outcomes and safety profiles, but the AMPC-TORe technique usually requires multiple endoscopic sessions [23][35].
Barola et al. performed a two-fold running suture TORe technique with a significant reduction in BMI (5.5 + 5.0%, p < 0.001 at mean follow-up of 113.2 ± 75.7 days (15.4%)); however, 15.4% of the patients developed a gastric stenosis that was treated with balloon dilation [27][39].
A new approach combining the restriction component of TORe followed by type 1 surgical distalization of the Roux limb may be another alternative for managing weight regain in high-BMI patients after RYGB; however, this could result in greater malabsorption, leading to greater deficiency syndrome [28][40].
More recently, rwesearchers have seen the emergence of a novel, modified technique: first performing an endoscopic submucosal dissection (ESD) before applying endoscopic sutures. This is known as ESD-TORe [25][26][37,38]. A retrospective study compared patients who underwent modified ESD-TORe vs. APC -TORe. At 12 months, the ESD-TORe group experienced greater weight loss compared with the traditional TORe group (12.1% ± 9.3% vs. 7.5% ± 3.3% TBWL) [26][38]. However, this technique resulted in a higher rate of major complications (21.1% for ESD-TORe vs. 8.77% for APC-TORe) which, combined with the technical difficulty of ESD, limits its widespread adoption [22][26][34,38].
On the other hand, the TORe procedure has demonstrated a high degree of safety, with only minor intraprocedural adverse effects (AE) such as superficial lacerations of the esophageal mucosa due to the use of the overtube [2][7][8][18][19][20][21][33][34][35][36][37][12,17,18,19,29,30,31,32,33,45,46,47]. Additional postprocedural serious AEs include bleeding from marginal ulceration and GJA stenosis [9][10][11][14][19][20][21][22][28][29][30][34][20,21,22,25,30,31,32,33,34,40,41,42]. In general, AEs can be successfully managed endoscopically without the need for surgery.
