Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Isabel Legaz and Version 2 by Camila Xu.
Degraded DNA fragments released into the blood or other fluids are known as cfDNA. Its first detection dates back to 1948 in patients with systemic lupus erythematosus.
- donor-derived cell-free DNA (cfDNA)
- graft injury
- acute rejection
- organ transplant
1. Introduction
In kidney transplantation, a biopsy is currently the gold standard for monitoring the transplanted organ. A biopsy involves taking a small tissue sample from the transplanted kidney, which is then examined under a microscope to assess organ health. This is done to assess for any signs of rejection or other problems that may be occurring in the transplanted kidney. However, this is far from an ideal screening method given its invasive nature and the discomfort it can cause the patient. Large-scale studies in renal transplantation show that approximately 1% of biopsies generate major complications, with a risk of macroscopic hematuria greater than 3.5% [1]. Most biopsy-related complications, such as pain and bleeding, are minor and localized, and can be managed conservatively [2]. The most severe complication, however, is the risk of perforation of the collecting system or the kidney itself, which can result in severe hemorrhage, sepsis, and even death. To minimize the risk of complications, careful patient selection, proper imaging guidance, and specialized instruments and techniques are essential [3][4][3,4]. Imaging techniques, such as ultrasound and computed tomography (CT), are essential for accurate needle placement. Ultrasound imaging is the most commonly used modality for needle guidance due to its versatility, cost-effectiveness, and relative safety. It allows for real-time visualization of the renal transplant and the surrounding anatomy, making it ideal for guiding percutaneous needle placement. CT imaging can also be used but is typically reserved for more complex cases with insufficient ultrasound imaging.
In addition, with current immunosuppressive therapies, the detection of subclinical rejection is too infrequent to justify this risk, which has meant that many units no longer perform these routine biopsies [5], thus raising the urgent need to find a new non-invasive biomarker that allows the detection of said rejection in order to intervene in time or modify immunosuppression. In response to this need, recent studies have shown that non-invasive biomarkers could be a viable option for detecting subclinical rejection [6][7][8][9][6,7,8,9]. These non-invasive biomarkers include urinary and serum markers such as urinary albumin-to-creatinine ratio (UACR) and donor-specific antibodies (DSA). Additionally, imaging techniques, such as magnetic resonance imaging (MRI) and ultrasound (US), have been used to detect graft changes that may signal rejection [10][11][12][10,11,12]. Finally, genetic and epigenetic biomarkers, such as microRNAs, have also been used to detect subclinical rejection [13][14][15][13,14,15]. Ultimately, using these non-invasive biomarkers could help identify and intervene in cases of subclinical rejection earlier, thus avoiding more serious complications.
2. Types de Cell-Free DNA
Degraded DNA fragments released into the blood or other fluids are known as cfDNA. Its first detection dates back to 1948 in patients with systemic lupus erythematosus [16][20]. It would not be until 1970 that this new biomarker would begin to be considered helpful for the clinic, as researchers observed differences in its concentration depending on the health status of the individual studied and began to see its application in cancer patients by allowing the detection of fragments of tumor DNA in the blood [17][21]. Its interest increased when it was discovered that tumor cells not only released cfDNA into the bloodstream but that these fragments also had the genetic and epigenetic changes of the tumor cells from which they had originated [18][22]. Shortly after, analysis of fetal cfDNA in maternal plasma began to be used to detect Rh mismatches and chromosomal aneuploidies [19][23]. Recent literature shows that different cfDNA types can be used as biomarkers of various disease states [20][21][16,24]. The following stand out for their relevance: ccf mtDNA (circulating cell-free mitochondrial DNA), ctDNA (circulating tumor DNA), cffDNA (cell-free fetal DNA), and dd-cfDNA (donor-derived cell-free DNA). These types and their applications are listed in Table 1.Table 1.
Different types of cfDNA and their main clinical applications.
| Type of Cell-Free DNA | Abbreviations | Potential Application |
|---|---|---|
| Circulating cell-free mitochondrial DNA | ccf mtDNA | Diagnostic and predictive markers in various disease states, markers of cell death, and non-specific tissue damage |
| Circulating tumor DNA | ctDNA | Marker in oncological diagnostics, monitoring of tumor development |
| Cell-free fetal DNA | cffDNA | Prenatal diagnostics, detection of fetal defects |
| Donor-derived cell-free DNA | dd-cfDNA | Evaluation of post-transplant complications |
3. History of Donor-Derived Cell-Free DNA
Non-patient cfDNA from a transplanted organ is known as circulating dd-cfDNA and can be detected in both blood and urine. Initially, the dd-cfDNA concentration increases to values greater than 5% after transplantation; however, these decrease rapidly and are practically undetectable after a week. Therefore, the presence of dd-cfDNA in the blood of the transplanted patient after a short period may be related to possible complications [1][23][1,26]. If the transplanted individual rejects the graft, the concentration of dd-cfDNA increases up to five times more than in healthy controls. In addition, these increased levels can be identified before any other clinical or biochemical symptom or complication, which is why it is of great interest for the early approach to subclinical rejection [24][27]. In 1998, this interesting biomarker was detected for the first time in the plasma and urine of solid organ recipients, identifying DNA from the Y chromosome in women transplanted with organs from male donors [24][25][27,28]. After this discovery, the technology began to evolve, emerging quantitative PCR techniques oriented to typing HLA genes. However, they had reproducibility problems and could not distinguish donor and recipient if they shared typings [5][26][5,29]. It would not be until 2011 that a method to detect dd-cfDNA employing digital PCR was devised based on analyzing the differences in SNPs between donor and recipient [27][30]. This strategy was optimized in 2016 with other authors [28][31] who, based on the principles of allelic imbalance, were able to measure dd-cfDNA levels by genotyping only the recipientm and between 150,000 and 600,000 SNPs from the donor, therefore, not being necessary to genotype the latter [15] altogether. Later that same year, other authors demonstrated that it was possible to quantify cfDNA levels by analyzing only 266 carefully chosen SNPs to minimize the probability that two unrelated individuals share them [29][32]. However, although these discoveries have laid the foundations for commercial dd-cfDNA detection kits, they have a series of limitations that have not yet been resolved, such as the impossibility of detecting dd-cfDNA levels in the transplantation of identical twins or differentiating the presence of more than two different genomes, as occurs in the case of retransplanted patients [20][30][16,33].4. Commercial Tests for dd-cfDNA Detection
Three commercial dd-cfDNA detection kits are available for clinical use in kidney transplantation; Allosure from CareDx, Prospera from Natera, and Trac from Viracor Eurofins [22][26][31][25,29,34]. The most commonly used and described in various publications is CareDX, where a panel of 266 SNPs in 22 somatic chromosomes was used to study 102 kidney transplant recipients, 27 with rejection confirmed by biopsy [30][33]. In the said test, a 1% dd-cfDNA was established as a cut-off to discriminate the presence or absence of active rejection, and it had a specificity of 85%, a sensitivity of 59%, a positive predictive value (PPV) of 61% and an 84% of accuracy negative predictive value (NPV). In this study, TCMR was insufficiently detected, as confirmed by Huang in an independent study with the same test [32][35]. One reason may be using relatively long amplicons (100–130 bp) in the employed test. According to Clausen et al. [33][36], the recommended amplicon length is 85.4 bp (66–103 bp). Prospera de Natera’s dd-cfDNA levels were determined in the following study by detecting 13,392 SNPs on four chromosomes [27][34][30,37], and a retrospective study was performed on 178 kidney transplant patients diagnosed with rejection. Acute T-lymphocyte-mediated (TCMR) or antibody-mediated (ABMR). Based on the results obtained, a cut-off for the presence of rejection more significant than 1% of dd-cfDNA was established, as in the Allosure method, with a specificity of 73%, a sensitivity of 89%, a PPV of 52%, and an NPV of 95% [35][38]. TCMR was well detected, presumably due to shorter amplicon size. Similar results were obtained in the Trifecta study [36][39]. These two studies would be the most relevant to date at the level of bibliographic background; however, a large number of companies are trying to develop dd-cfDNA kits, and that is where the Eurofins TRAC (Transplant Rejection Allograft Check) study would come in, which uses NGS techniques and recipient genotype data to determine the percentage of dd-cfDNA from the donated organ. In said study, biomarkers were determined in 77 kidney transplant patients with a cut-off of 0.70% to discriminate active rejection and sensitivity values of 58%, specificity of 85%, PPV of 55%, and NPV of 86% (Eurofins study) [35][38]. To clarify and inform the particular process, a schematic illustration of cfDNA isolation and analysis is shown in Figure 1.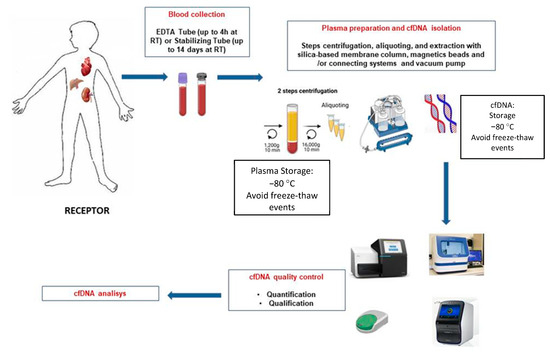
Figure 1.
Schematic overview of the different steps for blood sample processing of graft recipients and cfDNA extraction.
