Beyond its key role in calcium homeostasis, vitamin D has been found to significantly affect the cardiovascular (CV) system. In fact, low vitamin D levels have been associated with increased CV risk, as well as increased CV morbidity and mortality. The majority of effects of this molecule are related directly or indirectly to its antioxidative and anti-inflammatory properties. Generally, vitamin D insufficiency is considered for 25-hydroxyvitamin D (25(OH)D) levels between 21–29 ng/mL (corresponding to 52.5–72.5 nmol/L), deficiency as 25(OH)D levels less than 20 ng/mL (<50 nmol/L), and extreme deficiency as 25(OH)D less than 10 ng/mL (<25 nmol/L). However, the definition of an optimal vitamin D status, as defined by 25(OH)D, remains controversial for many extra-bone conditions, including CV disease.
- vitamin D
- 25(OH)D
- antioxidant
- determinants
- vitamin D status
- cardiovascular risk
- cardiovascular disease
1. Introduction
2. Vitamin D Metabolism
UVB sun rays are the main source of vitamin D, whereas less than 10% derives from dietary intake (e.g., salmon, mackerel and herring, mushrooms, eggs, and fish liver oil), but may be also added to other foods or available as a dietary supplement. Skin exposure to solar UV irradiation induces photolysis of a derivative of cholesterol (7-dehydrocholesterol) into pre-vitamin D3, then isomerized to vitamin D3 (cholecalciferol) [1]. As a liposoluble/hydrophobic molecule, vitamin D3 requires the binding with a transporter protein (vitamin D-binding protein, VDBP) to circulate in the blood. Then, it is hydroxylated in the liver to form 25(OH)D, the major circulating metabolite [1]. In the kidney, hydroxylation catalyzed by the 1alfa-hydroxylase enzyme produces the active hormone, 1,25-dihydroxy vitamin D (1,25(OH)2D), while 24-hydroxylase (CYP24) promotes the production of inactive forms [1]. When released by its binding with DBP to the tissues, 1,25(OH)2D mediates a number of actions through its intracellular vitamin D receptor (VDR). The main objectives are control of calcium and phosphorus homeostasis (kidney and intestine as principal target tissues) and bone health and turnover. Although 1,25(OH)2D represents the active form, there is general agreement on the measure of 25(OH)D as the best index of vitamin D status [2]. Notably, 25(OH)D has a higher concentration in the bloodstream with respect to the active form 1,25(OH)2D and shows a longer circulating half-life if compared to 1,25(OH)2D (3 weeks vs. 4 h, respectively), thus providing more representative information about the vitamin D status. Moreover, 1,25(OH)2D values are regulated through PTH (upregulation) and higher serum calcium and phosphate levels (downregulation). Therefore, because vitamin D deficiency may induce secondary hyperparathyroidism, 1,25(OH)2D may result in reduced, normal or even elevated despite evidence of vitamin deficiency.
3. Methodological Determinants
3.1. Preanalytical Issues
25(OH)D is generally measured in plasma or serum samples, although serum is the most used. In some cases, 25(OH)D levels were found to be higher in heparinized plasma than in serum samples or in ethylenediamine tetraacetic acid (EDTA) plasma [6][7][8][14,15,16]. When tubes with gel are used, no impediment for immunoassay evaluation was registered, whereas interferences have been observed with high-performance liquid chromatography (HPLC) or mass spectrometry approaches [6][9][14,17]. Different storage conditions (fresh samples vs. up to 24 h at room temperature, different centrifuging times/temperature, multiple freeze–thaw cycles) did not significantly affect 25(OH)D values [6][8][9][10][11][12][7,14,16,17,18,19]. Long-term stability of 25(OH)D at −20 °C and −80 °C is generally acceptable, although a 15% variation after two months [13][20] or significant loss after four months at −20 °C was observed [6][14]. In addition, a variation of 25(OH)D levels after five years of storage also at low temperature (−80 °C) has been reported, which may be taken into consideration for studies involving sample long-term storage [14][21].3.2. Analytical Issues
There are several different analytical assays available for the determination of 25(OH)D, which include immunoassays (e.g., chemiluminescence immunoassay-CLIA and radioimmunoassay-RIA, high-performance liquid chromatography-HPLC with UV/fluorescence detection, liquid chromatography-mass spectrometry-LC-MS or tandem mass spectrometry-LC-MS/MS) [15][16][22,23]. Although LC-MS/MS maintains high analytical performance, aspects related to the high cost of instruments, time-consuming, limited throughput, and complexity of methodological problems requiring skilled professional staff, greatly limit the inclusion of this technique in clinical practice. Thus, the introduction of automated immunoassays has enabled rapid uptake of testing and the ability to respond to an ever-increasing demand for vitamin D testing (with the exception of RIA, due to available alternatives that avoid radiolabeled compounds). Nonetheless, these assays still present a highly variable analytical performance, and many cross-reactivity problems with several vitamin D metabolites as well as matrix effects (e.g., heterophilic antibodies) [14][21].4. Environmental Determinants, Lifestyle Habits, and Skin Pigmentation Affecting 25(OH)D Status
A variety of biological and environmental factors can influence vitamin D status in humans [17][39]. (Table 12). Synthesis of vitamin D in human skin occurs under ultraviolet exposure, thus any factor (e.g., season, latitude, time of day, cloud, ozone) influencing ultraviolet radiation levels, may significantly affect vitamin D production [18][19][40,41]. The day length, which is the period between sunrise and sunset, is dependent on latitude and time of the year, thereby peak levels of 25(OH)D are recorded following the summer months [20][21][42,43].| Environmental Determinants | Anthropometric Determinants | Life-Style Determinants |
|---|---|---|
sunlight exposure: intensity and duration season latitude length of day presence of clouds air pollution/ozone |
aging race/phototype gender body mass index/obesity genetic asset: presence of specific polymorphisms hepatic/renal dysfunction pregnancy |
dietary intake supplementation/fortified foods sunscreen/clothes time spent outdoor/outdoor sports |
5. Anthropometric Characteristics
Differences in age together with other factors, including vitamin D receptor gene polymorphisms, and constitutive skin pigmentation are responsible for a not negligible part (up to 15%) of the interpersonal variation in the UVB-induced 25(OH)D synthesis in the skin [30][73]. It is known that the ability to produce vitamin D3 is impaired in the elderly, with an aging-related progressive reduction in skin levels of 7-dehydrocholesterol [31][55]. This effect can be further exacerbated by reduced nutritional intake of vitamin D, increasing adiposity, less exposure to sunlight due to immobility, and staying indoors, all common factors in adult aging [31][32][55,74]. Additionally, as the kidney plays a central role in the regulation of vitamin D metabolism and circulating levels, a reduced renal function can lead to the inhibition of renal 1α-hydroxylase expression, upregulation of 24-hydroxylase and 1,25(OH2)D degradation, and the resulting vitamin D deficiency observed among patients with chronic kidney disease or undergoing dialysis [33][34][75,76]. Vitamin D status could also vary according to sex, although there is contrasting evidence as regards this association [35][77]. In this context, Muscogiuri et al. (2019) found that 25(OH)D levels were lower in females than males across all body mass index (BMI) categories [35][77]. 25(OH)D is lipophilic and it has been estimated that about 17% of orally-administered vitamin D dose is stored in adipose tissue and the rest is consumed or metabolized, indicating that adipose tissue acts both as a storage and buffering site of vitamin D [36][37][80,81]. Accordingly, clinical studies have found that obese individuals have a greater risk (35–40%) of vitamin D deficiency, regardless of age and latitude [38][39][40][82,83,84]. Vitamin D has been shown to affect adipocyte development, although results from in vitro and in vivo studies assessing the effect of vitamin D in adipogenesis are conflicting [37][41][81,85].6. Vitamin D and Genetic Determinants
Besides environmental and nutritional aspects, circulating levels of vitamin D are also influenced by genetic patterns. Many investigations on the causal role between vitamin D and several diseased conditions relied on studies on identical and non-identical twin pairs, which normally have similar trait-relevant environments [42][87] and allow better esteem taking into account genetics and/or environmental effects. These studies have shown that vitamin D concentrations are highly heritable, between 29% and 86% [42][43][44][87,88,89]. In general, the considerable variance in the evaluation of vitamin D heritability depends on many different reasons, such as age, gender, seasonality, and comorbidities [45][90]. Therefore, well-powered twin studies with reliable controls are fundamental in the determination of genetic contribution to the vitamin D circulating component. Over time, the studies of genetic determinants associated with the modification of vitamin D circulating levels in humans proceeded through several stages. The first classical approach was the linkage analysis of specific genetic intervals on the chromosome and their relation to the disease [46][47][91,92]. In general, linkage analysis conducted on affected subjects of the same family unveils which locus segregates with certain disease/phenotype; therefore, this approach may help to identify which chromosome region(s) may be associated with vitamin D variability in many diseases [46][91]. Differently from the linkage analysis-based studies, the candidate gene approach and genome-wide association study (GWAS) allowed the identification of reliable and reproducible associations with vitamin D circulating concentration. By candidate gene studies, it is possible to define if the frequency of a specific variant (single nucleotide polymorphism or gene) is associated with the variation of vitamin D levels, usually in the context of unrelated subjects. Several genes, closely related to vitamin D metabolism, have been studied: CYP2R1 and CYP27B1, involved in vitamin D hydroxylation [48][93]; GC, encoding for a vitamin D carrier protein [49][94]; VDR, coding for Vitamin D receptor [49][94]; CYP24A1, a cytochrome P450 gene [50][95]. GWAS constituted the major advancement in the identification of novel links between specific diseases and their biological determinants. Instead of focusing on a limited number of specific gene variations as in candidate gene studies, this innovative approach, which relies on the haplotype map of the genome and array-based advanced technology, rapidly explores the whole genome. The first GWAS of vitamin D consisted of 1012 related subjects from the Framingham Heart Study and genotyped 70,987 SNPs. However, because of the limited power and coverage of the analysis, none of the SNPs has passed the genome-wide significant p threshold at 5 × 10−8, obtained from Bonferroni’s correction for multiple testing [51][96]. Ultimately, important key findings on vitamin D genetics were obtained though large-scale international efforts to perform GWAS meta-analyses, involving large numbers of individuals from different cohorts, so as to obtain sufficient statistical power to reliably identify an association between circulating vitamin D levels and genetic determinants of several health outcomes [52][97].7. Vitamin D Mechanisms Related to Its Antioxidant/Antiinflammatory Action and Vascular Health
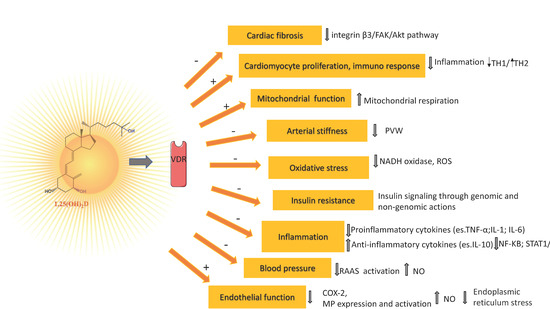
If a causal role of vitamin D for bone health is widely recognized, with vitamin D deficiency associated with most cases of rickets and osteomalacia, a number of genetic, molecular, cellular, and animal studies strongly suggest that vitamin D signaling has many extraskeletal effects, including regulation of cell proliferation, immune and muscle function, skin differentiation, and reproduction, as well as vascular and metabolic properties, which are not strictly related to calcium homeostasis [53][54][102,103]. On the other hand, these extraskeletal effects are characterized by some controversies due to conflicting results between observational and interventional studies [55][104]. Nonetheless, it is well established that vitamin D is able to modulate immune response (Th1/Th2 reduction) [56][105], while vitamin D deficiency has been associated with numerous conditions (e.g., multiple sclerosis, type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, hepatitis, asthma, respiratory infections) and with an increased risk of any type of cancer and a reduced survival rate [55][57][104,106]. The biological effects of vitamin D are mediated by VDR, a member of the transcription factor superfamily of nuclear receptors which, upon activation by its binding to the active form of vitamin D and to a retinoid X receptor, translocate to the nucleus where it may regulate the transcription of vitamin D-sensitive target genes within hours or days [58][107]. Nitric oxide (NO), produced in the endothelium by endothelial NO synthase (eNOS), in addition to its potent vasodilatory effect, protects the vessels from developing atherosclerosis [59][115]. Experimental studies reported the ability of vitamin D to stimulate NO production via a direct increase of eNOS gene expression and activation of eNOS in intracellular calcium-dependent pathways (Figure 1) [60][61][116,117]. Vitamin D elicits a vasoprotective effect also through a decrease of oxidative stress (a major indicator of NO bioavailability and cause of damage to protein, lipids, and DNA), by upregulating expression of antioxidative enzymes and activating the nuclear factor erythroid 2-related factor 2 antioxidant pathway (Figure 1) [62][63][64][118,119,120].