Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Ernesto Soto.
Terpenes and essential oils are materials of great commercial use due to their broad spectra of antibacterial, antifungal, membrane permeation enhancement and antioxidant biological properties, as well as for their use as flavors and fragrances. Yeast particles (YPs) are 3–5 µm hollow and porous microspheres, a byproduct of some food-grade yeast (Saccharomyces cerevisiae) extract manufacturing processes, that have been used for the encapsulation of terpenes and essential oils with high payload loading capacity (up to 500% weight) and efficiency, providing stability and sustained-release properties.
- yeast particle
- terpenes
- essential oils
- microencapsulation
- sustained release
1. YP–Terpene with a Hydrocolloid Plug Seal
Hydrogels are high-water content materials prepared by crosslinking polymers and have applications in encapsulation technologies due to their ability to trap and slowly release the target payload [1][2]. Several non-toxic, biocompatible, and biodegradable compounds such as the natural polysaccharides chitosan and alginate have been extensively studied in drug encapsulation. The use of hydrocolloids can be applied in YPs by loading of the hydrocolloid polymer precursor and crosslinking to form a shell embedding the payload and plug sealing the pores of YPs. This approach has been previously demonstrated by plug-sealing yeast particles encapsulating the TB drug rifampicin using alginate–calcium and chitosan hydrocolloids [3].
Hydrocolloid plug-sealed YP–terpene samples have been prepared using calcium crosslinked alginate. Alginate plug-sealed YP–terpene samples extend the duration of terpene release for a few hours compared to YP–terpenes. Limitations of this approach are the need for a rapid crosslinking reaction to plug seal the YP pores and prevent dissolution of the polymer matrix in the terpene payload.italics
2. YP–Terpenes with a Calcium Carbonate Plug Seal
The use of inorganic amorphous insoluble materials prepared in situ in YPs is an approach, similar to the use of hydrocolloids in which the YP pores are plug sealed by the inorganic matrix slowing diffusion of terpene release. Calcium carbonate can be formed in situ in YPs by absorption of calcium chloride and subsequent reaction with sodium carbonate or sodium bicarbonate at pH 9–10. This encapsulation method also extends the duration of terpene release for a few hours compared to YP–terpenes. The short extension of terpene release with hydrocolloid or carbonate plug seal methods over the first-generation YP–terpene technology limits their application. Encapsulation approaches with better control of payload release and that significantly extend the duration of terpene release from the particles are described in the following sections.
3. YP In Situ Encapsulation of Terpenes in Polyurethane/Polyurea Nanoparticles
Nanoparticles can be used in combination with YPs by either physisorption or chemical absorption by non-covalent or covalent binding of large nanoparticles (average diameter > 40 nm) to the outer surface of YPs, encapsulation of small nanoparticles (average diameter < 30 nm) inside the hollow cavity of YPs, or by in situ formation of nanoparticles in YPs [4][5][6]. Polyurethane/polyurea nanoparticle drug delivery systems are formed by the reaction of isocyanates and diols on the surface of an emulsion containing target drug [7][8].
YP–terpene polyurethane nanoparticles can be formed in situ in YPs by co-loading of terpene with the polyol/polyamine precursor and then crosslinking with an isocyanate reagent (e.g., isophorone diisocyanate, toluene diisocyanate).
This YP–terpene encapsulation approach achieves high encapsulation efficiency, similar to YP–terpenes 1.1:1, and significantly decreases the rate of terpene release (Figure 3B1). However, the approach has limitations due to the need to use toxic isocyanate crosslinkers, possible side crosslinking reactions of polyurethane with terpenes containing hydroxyl groups, and regulatory concerns with the application of polyurethane materials in agricultural products or for direct human health applications. However, this YP–terpene encapsulation approach has potential if developed using green materials such as non-isocyanate polyurethane nanoparticles [9].
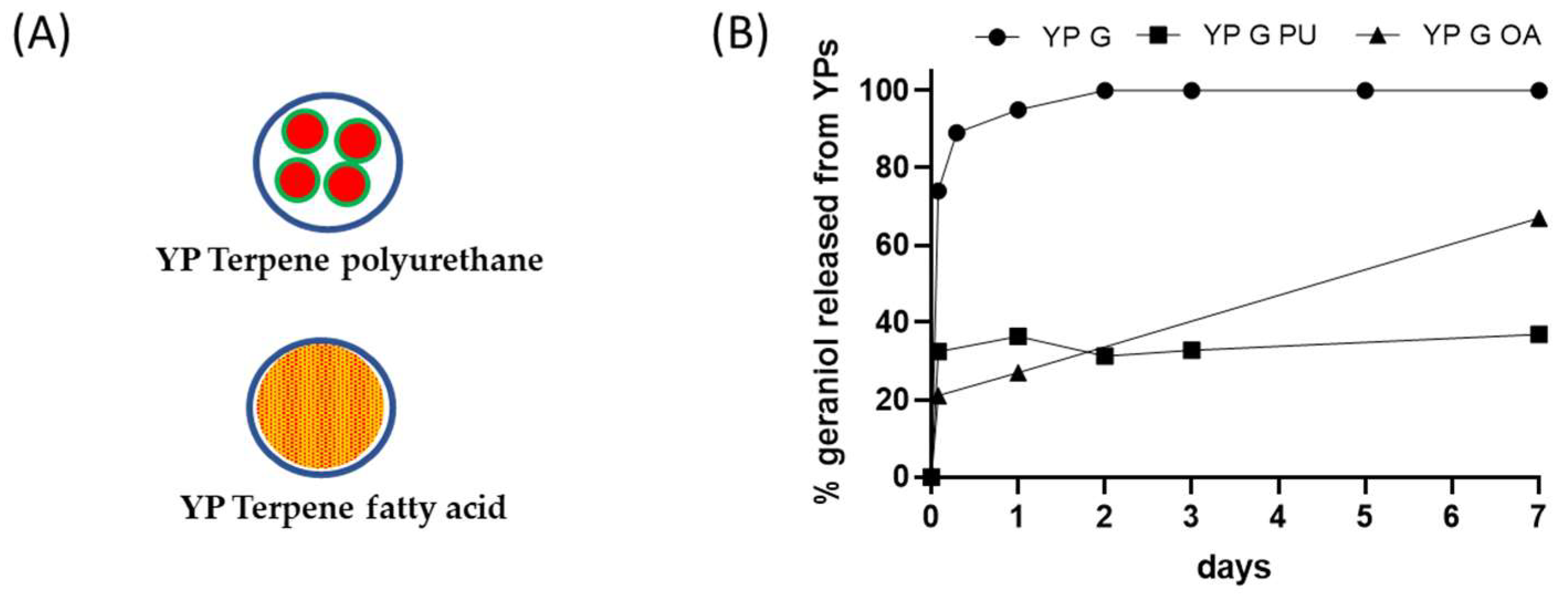
Figure 31. (A) Schematics of YP–terpene formulations prepared by encapsulation of terpene in polyurethane or co-loading of terpene with a fatty acid, and (B) kinetics of geraniol release from YP samples at 25 °C in YP–terpene suspensions at total geraniol concentration of 1 mg/mL (YP G: YP geraniol, YP G PU: YP geraniol polyurethane, YP G OA: YP geraniol oleic acid).
4. YP Lipid Terpenes
Fatty acids can be used as sequestering agents of terpenes inside YPs. The rationale in this approach is to use fatty acids that (1) dissolve the target terpene compound and (2) are more hydrophobic than the terpene. The materials can be co-loaded in a single step or sequentially loaded in YPs. This approach generally requires loading of fatty acid and terpenes at a >1:1 fatty acid:terpene weight ratio to maximize terpene retention in the hydrophobic lipid environment. The selection of lipids with melting point above room temperature improves the stability of the YP–terpene lipid material by forming a solid matrix inside the cavity of YPs. The results in Figure 31B show significant reduction in the release of geraniol from a YP sample containing geraniol and oleic acid (1:1 oleic acid:geraniol) compared to the YP geraniol control. The geraniol release from YP–geraniol–oleic acid shows a burst release (~30% geraniol) between 0 and 4 h, and the remaining geraniol remains stably encapsulated up to 7 days (Figure 2B). These YP lipid terpene materials have potential use for long-term sustained terpene-release applications.
5. Hyper-Loaded YP–Terpenes
The first-generation YP–terpene formulations were developed to encapsulate up to a 2:1 w/w terpene:YP ratio. Recently, methods to increase terpene loading capacity in YPs up to 5:1 w/w were reported in the literature [10]. The preparation of hyper-loaded YP–terpenes is possible by carefully controlling the ratio of water to YP to minimally hydrate the porous shell, allowing for slow diffusion of terpene payload. The hyper-loading of terpenes in YPs leads to an increase in YP diameter from 5.4 µm (empty YPs) to 7.7 µm (YP–terpene 5:1) to accommodate the large terpene droplet at higher payload levels in the swollen hollow cavity of the particles (Figure 42A–C). In addition to increasing efficient payload loading capacity, these hyper-loaded YP–terpene samples provide for enhanced sustained terpene release, up to three-fold compared to the previously developed YP–terpene 1.1:1 formulation (Figure 42D) and show higher antimicrobial potency than unencapsulated terpenes. The original work also described methods to improve thermal terpene storage encapsulation stability by producing hyper-loaded YP–terpene samples in a solvent mixture of 70% water–30% glycerin at 100 g YP/L and water-suspended terpene concentrations from 300 to 450 g/L.
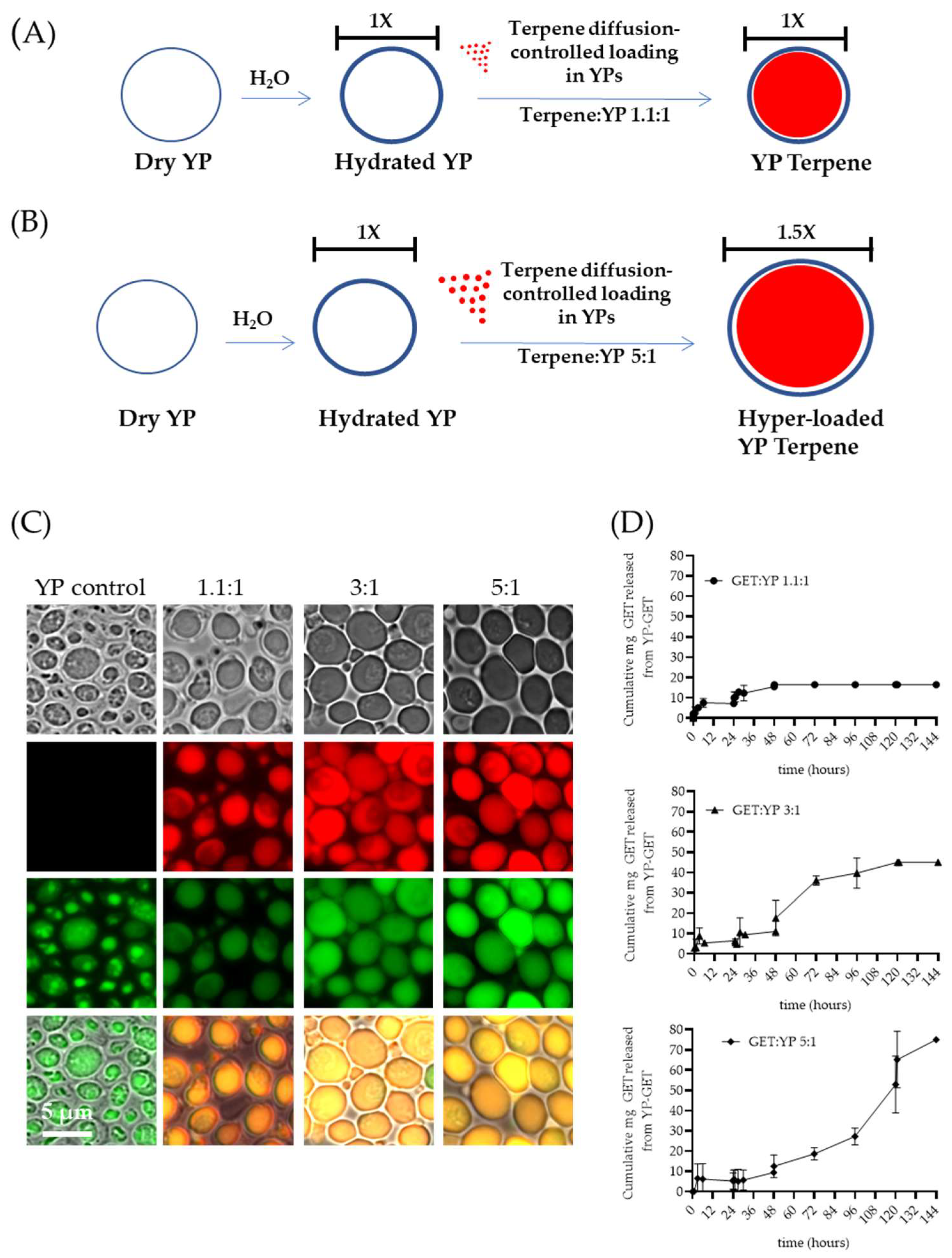
Figure 42. Schematics of diffusion-controlled terpene loading in YPs at terpene:YP ratios of (A) 1.1:1 and (B) 5:1. (C) Microscopy images showing FITC–concanavalin A stained YPs and Nile red stained terpenes in empty YP control and YP GETs loaded at GET:YP ratios of 1.1:1, 3:1, and 5.1. (D) Cumulative GET release from YPs showing extension of wetting/terpene release cycles in YP GET 1.1:1 and hyper-loaded YP GET at ratios of 3:1 and 5:1 GET:YP. Figures adapted from Reference [10] and reproduced with permission from MDPI.
The hyper-loaded YP–terpenes previously reported in the literature were prepared as homogenized liquid suspensions. It is also possible to extrude the hyper-loaded YP–terpene suspension to produce dry hyper-loaded YP–terpene granules (Figure 53A). Samples of YP GET suspensions hyper-loaded at GET:YP weight ratios of 4:1 and 5:1 were extruded to form YP GET granules. The dry granules were characterized for encapsulation efficiency and kinetics of terpene release. The results show that ~60–70% of GET was retained inside YPs during the extrusion and drying steps (Figure 53B). The kinetics of release from YP GET 1:5 granules was evaluated under three conditions: (1) granules that were suspended in water and sonicated, resulting in rapid disaggregation of granules into single YPs, (2) granules suspended in water and mixed with gentle agitation, and (3) granules suspended in water and not mixed (static). The results in Figure 42C show the effect of mixing on the release of GET from YP GET granules, samples that were sonicated showed burst GET release, YP GET granules that were mixed with mild agitation released payload in ~48 h, and samples that were not mixed only released up to 20% GET after 5 days of incubation in water at a target GET concentration of 1 mg/mL.
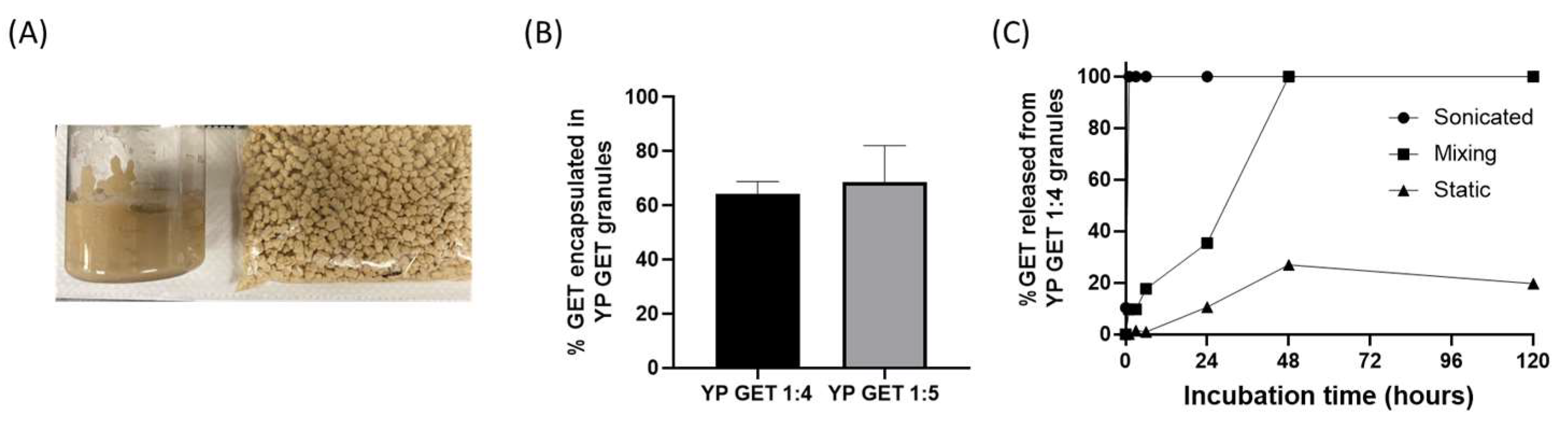
Figure 53. (A) Picture of homogenized aqueous suspension of YP GET 1:4 and YP GET 1:4 granules, (B) encapsulation efficiency of GET 2:1:2 in YP GET granules, and (C) GET release from YP GET 1:4 granules suspended in water at 1 mg GET/mL at room temperature under three incubation conditions.
The development of hyper-loaded YP–terpenes has a wide range of potential agricultural and pharmaceutical applications that could benefit from a delivery system with a high payload loading capacity combined with increased payload stability and sustained release properties.
6. YP–Terpene with a pH-Sensitive Eudragit® Coating
Eudragit® is a brand name of polymethacrylate-based copolymers. These copolymers are composed of methacrylic acid and methacrylic/acrylic esters and are modified to include anionic, cationic and/or neutral charge. The polymers are responsive under different pH conditions and are extensively used in pharmaceutical coatings for taste-masking and targeted delivery [11][12].
WThe researchers evaluated the coating of YP carvacrol with Eudragit® L-100, a polymer that forms an insoluble coat below pH < ~5.5 and dissolves at pH > 5.5 and is used in pharmaceutical coating of pills for oral delivery of drugs to the intestine. It was not possible to efficiently coat YP carvacrol or other terpenes with Eudragit® L-100 or other Eudragit® polymers, as the terpene dissolves the coat regardless of pH. This limitation was overcome by coating a mixture of carvacrol with undecanoic acid (UDA), as the fatty acid acts as a sequestering agent of carvacrol, preventing the terpene from dissolving the coat. Samples of YP carvacrol–UDA–Eudragit® L-100 and YP carvacrol control were incubated in simulated digestion conditions: first, the samples were incubated in simulated gastric fluid (SGF, pH 1.5) with pepsin for 2 hours at 37 °C; the samples were centrifuged, and the SGF was collected for HPLC analysis of released carvacrol; the YP pellets were suspended in simulated intestinal fluid (SIF, pH 6.8) with pancreatin at 37 °C; and the SIF was also analyzed for released carvacrol after 2 h incubation. The results in Figure 64B show that coated samples were effective at reducing carvacrol release in SGF, and they released the payload in SIF after dissolution of the Eudragit® coat. These results are encouraging, as this approach offers the possibility of selectively targeting terpenes to the intestine. However, this approach has the limitation that the use of fatty acid and Eudragit® L-100 reduces the loading capacity of terpenes in YPs.
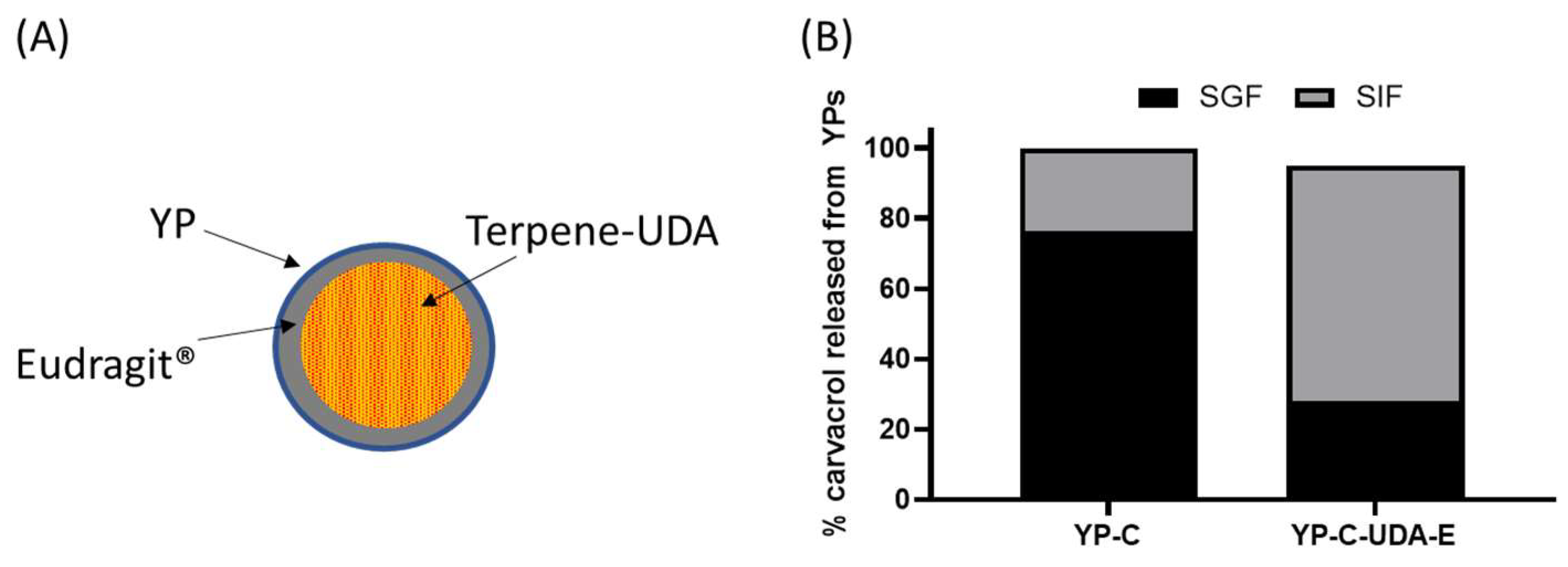
Figure 64. (A) Schematic of a YP–terpene–UDA particle coated with Eudragit® polymer and (B) carvacrol released from YPs in YP carvacrol (YP-C) control sample and YP carvacrol co-loaded with UDA and coated with Eudragit® L-100 (YP-C-UDA-E). The release assay was performed by sequential incubation of YP samples in simulated gastric fluid (SGF) at 37 °C for 2 h followed by incubation in simulated intestinal fluid (SIF) at 37 °C for 2 h. Carvacrol concentration during simulated digestion was 1 mg/mL.
7. YP Pro-Terpene with Stimuli-Controlled Terpene Release
A method to use YP for delivery of terpenes with stimuli-responsive control of terpene release was recently developed [13][14]. Biodegradable pro-terpene compounds were synthesized (Figure 75A) and encapsulated in YPs (Figure 75B) at a target weight ratio of 1:1 terpene:YP (~1.7–1.9 pro-terpene:YP weight ratio). In one pro-carvacrol-YP example formulation (Figure 75C), the kinetics of carvacrol release at pH 7, 37 °C, in suspensions at 1 mg carvacrol/mL show that it takes up to 16 days for YP pro-carvacrol to completely release its carvacrol content. The YP carvacrol control completely released carvacrol in < 1 d. The kinetics of terpene release from YP pro-carvacrol increased at higher pH (complete release in < 2 h at pH 10) or at acidic or neutral pH in the presence of esterases that hydrolyze the scaffolded terpene compound. In addition to the stimuli-responsive release of terpenes, the YP pro-terpenes show higher encapsulation stability than YP–terpenes due to pro-terpenes being non-volatile solids at room temperature.
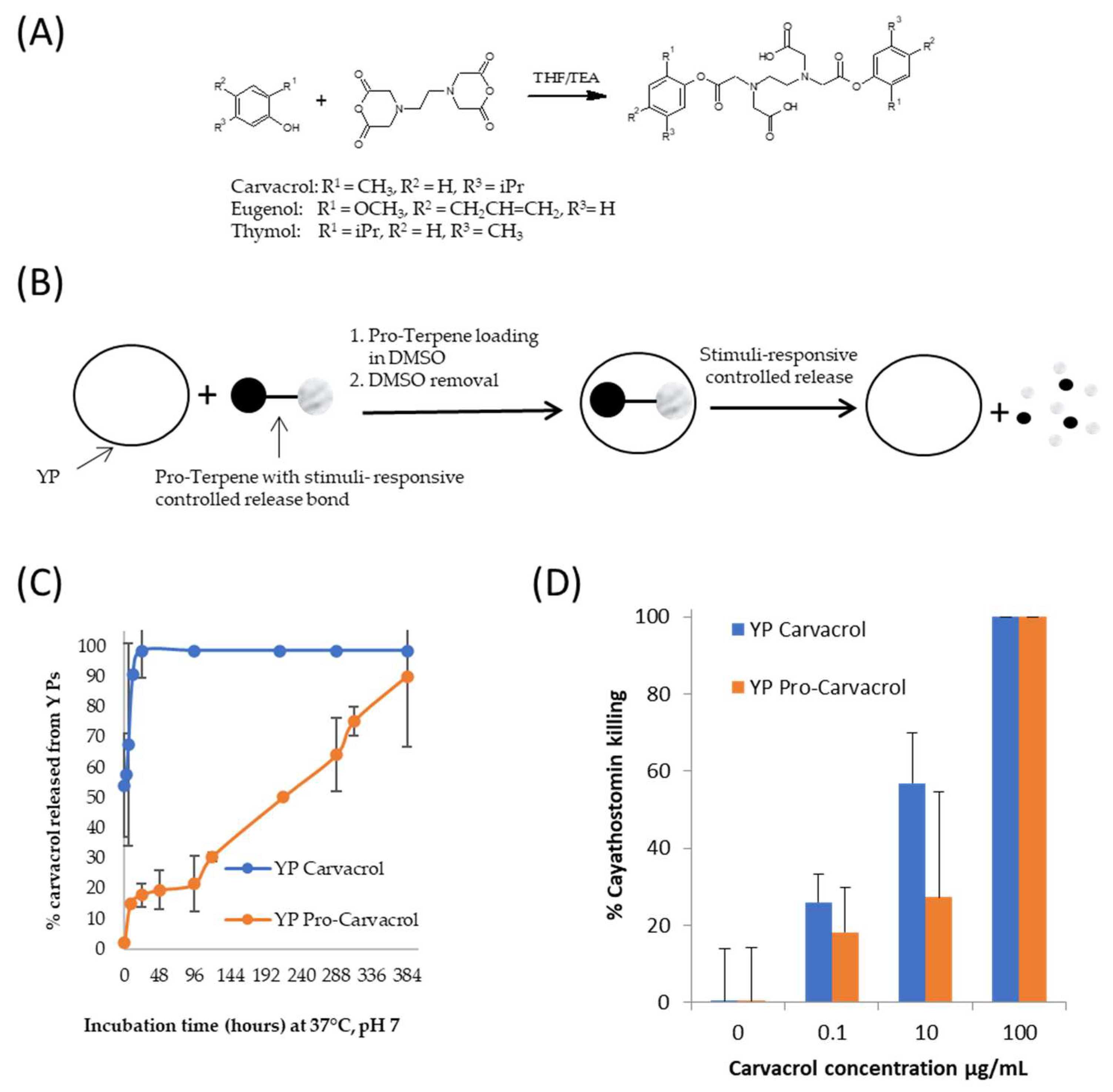
Figure 75. (A) Synthesis of pro-terpenes from parent terpene compounds (eugenol, carvacrol, thymol) and EDTA dianhydride, (B) schematics of pro-terpene loading in YPs and stimuli-controlled terpene release, (C) kinetics of carvacrol release from YP carvacrol and YP pro-carvacrol in 0.1 M phosphate buffer saline (PBS, pH 7) at 37 °C at a concentration of 1 mg carvacrol/mL, and (D) in vitro activity of carvacrol samples in cyathostomin egg-to-larvae assay. Figures adapted from Reference [14] and reproduced with permission from MDPI.
The YP pro-terpenes retained the full biological activity of the parent terpene compound in antibacterial, antifungal, and anthelmintic assays. The results in Figure 75D are an example of retention of biological in vitro activity showing that YP pro-carvacrol had similar efficacy as YP carvacrol in a cyathostomin egg-to-larvae assay. These YP pro-terpene samples have potential for development in pharmaceutical applications due to their improved stability over YP–terpenes and stimuli-responsive controlled release.
Yeast particles can be used for the encapsulation of terpenes and essential oils with high payload capacity and efficiency. The use of YPs allows for encapsulation levels up to 5:1 terpene:YP weight ratio; this high encapsulation capacity is achieved without the use of alcohols and potentially toxic surfactants typically required to stabilize terpenes in different formulations.
References
- Hariyadi, D.M.; Islam, N. Current status of alginate in drug delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095.
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17.
- Soto, E.R.; Kim, Y.S.; Lee, J.; Kornfeld, H.; Ostroff, G.R. Glucan particle encapsulated rifampicin for targeted delivery to macrophages. Polymers 2010, 2, 681–689.
- Soto, E.R.; Ostroff, G.R. Glucan Particles as Carriers of Nanoparticles for Macrophage-Targeted Delivery. In Nanomaterials for Biomecidine; American Chemical Society: Washington, DC, USA, 2012; pp. 57–79.
- Soto, E.R.; O’Connell, O.; Dikengil, F.; Peters, P.J.; Clapham, P.R.; Ostroff, G.R. Targeted Delivery of Glucan Particle Encapsulated Gallium Nanoparticles Inhibits HIV Growth in Human Macrophages. J. Drug Deliv. 2016, 2016, 8520629.
- Soto, E.R.; Caras, A.C.; Kut, L.; Castle, M.; Ostroff, G.R. Glucan Particles for Macrophage Targeted Delivery of Nanoparticles. J. Drug Deliv. 2012, 2012, 143524.
- Morral-Ruíz, G.; Solans, C.; García, M.L.; García-Celma, M.J. Formation of pegylated polyurethane and lysine-coated polyurea nanoparticles obtained from O/W nano-emulsions. Langmuir 2012, 28, 6256–6264.
- Cheng, J.Y.; Hou, T.Y.; Shih, M.F.; Talsma, H.; Hennink, W.E. Polyurethane-based drug delivery systems. Int. J. Pharm. 2013, 450, 145–162.
- Bossion, A.; Jones, G.O.; Taton, D.; Mecerreyes, D.; Hedrick, J.L.; Ong, Z.Y.; Yang, Y.Y.; Sardon, H. Non-Isocyanate Polyurethane Soft Nanoparticles Obtained by Surfactant-Assisted Interfacial Polymerization. Langmuir 2017, 33, 1959–1968.
- Soto, E.R.; Rus, F.; Ostroff, G.R. Yeast Particles Hyper-Loaded with Terpenes for Biocide Applications. Molecules 2022, 27, 3580.
- Patel, I.; Ajaykumar, T.; Srivastav, B. Eudragit a versatile Polymer: A Review. Int. J. Chem. Pharm. Sci. 2011, 1, 152–164. Available online: http://www.pharmaresearchlibrary.com/wp-content/uploads/2013/08/PRL2013-IJCPS-1783.pdf (accessed on 22 January 2023).
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149.
- Soto, E.R.; Rus, F.; Ostroff, G.R. Yeast Cell Wall Particle Encapsulation of Pro-Terpene Payloads. 2019. Available online: https://briefs.techconnect.org/papers/yeast-cell-wall-particle-encapsulation-of-pro-terpene-payloads/ (accessed on 5 November 2021).
- Soto, E.R.; Rus, F.; Li, H.; Garceau, C.; Chicca, J.; Elfawal, M.; Gazzola, D.; Nielsen, M.K.; Urban, J.F., Jr.; Aroian, R.V.; et al. Yeast Particle Encapsulation of Scaffolded Terpene Compounds for Controlled Terpene Release. Foods 2021, 10, 1207.
More
