Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Marianna Trebuňová and Version 2 by Rita Xu.
The growing importance of regenerative medicine and tissue engineering (TE) reflects the fact that bone metabolic and related diseases represent approximately 50% of all chronic diseases for people above the age of fifty. In addition, mechanical damage of bone often occurs because of an accident, required surgery and so forth. Bone defects or bone injuries caused by aging, traffic accidents, fractures, or bone tumor resection are among the serious problems in orthopedics because they cause major damage to health and lower the quality of life.
- biodegradability
- scaffold
- implant
1. Introduction
Internal fixation is required for reconstructive surgery on fractured bone to maintain the anatomic reduction in the fragments and provide stability during the healing process. In the past, bone fractures were fixed by the methods of applying metal implants. To substitute the metal implants for internal fracture fixation, numerous biodegradable materials (BMs) were developed. Biodegradable implants are increasingly used in regenerative medicine and sports medicine [1]. To be used successfully for fracture fixation, BMs must have sufficient strength and not degrade too rapidly. In an ideal scenario, these implants would break down as the wound healed, transferring load gradually to the healing tissue.
Today’s regenerative medicine and tissue engineering are using a large portfolio of BMs, which are used largely as substitutes for damaged or missing hard tissue. Natural and synthetic biodegradable polymers and hydrolysable metals make the main components for the creation of temporary medical implants [2]. Recently, much attention has been paid to materials based on extracellular matrix (ECM) [3][4][3,4], which consist of proteins, glycosaminoglycans and glycoproteins [5]. There is no doubt that the development and application research of BMs has significantly intensified in the last decade, as evidenced by the growing number of publications in this area.
2. Indications and Materials for Biodegradable Implants in Orthopedics
There are several typical clinical indications for the use of biodegradable implants in orthopedics, which are mostly used for fractures stabilization, osteotomy procedures, bone grafts and fusions [6][7][6,7]. Furthermore, they can be used in re-attachment of tendons, ligaments, meniscal cracks, and other tissue structures [8]. The most common indications for biodegradable implants in orthopedics include anterior cruciate ligament reconstruction, meniscus repair, and ankle fracture treatment [9]. The occurrence of clinical indications for biodegradable implants is comprehensively demonstrated in Figure 1.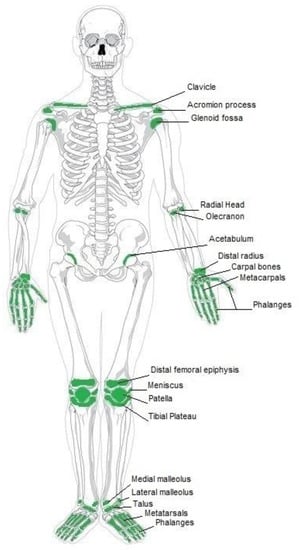
Figure 1. Current clinical applications of biodegradable implants.
3. Smart Biodegradable Materials for Tissue Engineering
Traditionally, biodegradable materials were designed to interact with living tissue temporarily or permanently to provide functions, such as mechanical support. Smart BMs are defined as those that respond to external stimuli, such as light, magnetic fields, ultrasound, etc. Typical smart BMs include, e.g., photoresponsive and chemoresponsive polymers that combine sensing and actuation within the same material, without need for external devices [41][42][43][119,120,121]. Moreover, development of smart bioactive glasses for bone contact applications is becoming a hot research area in TE [44][122]. Recent research in this domain focuses on the molecular interaction of bioactive glass-based ionic dissolution products with their physiological surrounding environment [45][123]. Another example of smart biomaterial is decellularized extracellular matrix, which is the noncellular component of tissue that retains relevant biological cues for cells [46][124]. The related research is oriented towards directly using the component of the dECM to obtain scaffolds simulating native ECM [47][125]. Montoya et al. [48][126] suggested classifying smart biomaterials according to their degree of interaction with their external environment and the subsequent biological responses to clarify the concept of smartness in this context. The authors categorized smart materials into three kinds, namely, active, responsive, and autonomous. The inert biomaterial is just biocompatible or bioinert, while the active one can provide planned one-way interaction, e.g., bioactive therapy, with biological tissue. One of the first materials of this category was bioactive glass composed of four oxides, namely SiO2-CaO-Na2O-P2O5, introduced by Hench [49][127]. The main limitation of active biomaterials lies in the limited duration and efficacy of the therapy due to their degradation in a biological environment. Active biomaterials, namely polymer and lipid-based ones are often used for the controlled release of drugs like antibiotics, antiseptics, vitamins, and statins [50][51][128,129]. Responsive biomaterials can receive a stimulus and provide feedback to it through triggered reactions. Examples of such materials are artificial cells and hydrogels [52][130]. Especially, the need for biodegradable hydrogels in biomedical applications is significant since their physical properties can be designed to follow those of articular cartilage [53][131]. Recent developments in the design of responsive nanocomposite hydrogels increase their potential in biomedical applications including their utilization as therapeutic platforms for the delivery of precisely prescribed medications [54][132]. The responsive functionalities of biomaterials can be triggered from internal or external sources. The stimuli coming from inside an organism are internal, while external sources generate stimuli from outside of the body like heat, light, chemicals, or pressure. Both kinds of the signals can be categorized into three groups: biological, chemical, and physical [55][133]. For instance, PLLA-based biomaterials processed into piezoelectric structures can be engineered as scaffolds for promoting cellular growth during electrostimulation [56][134]. The low piezoelectric effect of PLLA is similar in magnitude to that of natural biomacromolecules like collagen [57][135] giving it the ability to interact with biological systems without being rejected [58][136]. The highest degree of smartness represents biomaterials capable of autonomously responding to the surrounding environment. Biomaterials with such properties can be considered as kind of dynamic biomaterials, which respond to stimuli by autonomous feedback loops [52][130]. The model of autonomous biomaterial is graphically illustrated in Figure 2.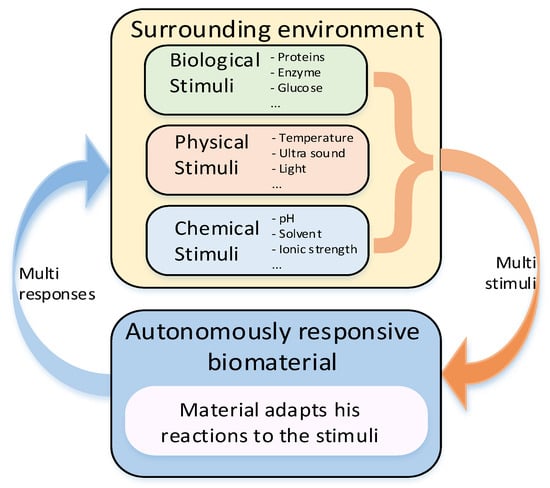
Figure 2. Model of stimuli-responsive biomaterial with closed loop.
