Magnetic resonance-guided radiotherapy (MRgRT) is an emerging radiotherapy technology combining real-time magnetic resonance imaging and radiation delivery. By administering radiation with a linear accelerator with built in low-field or high-field MRI, practitioners have a greater ability to align to the target for daily set-up, precisely track the motion of and thereby target or avoid tissues and adapt to inter-treatment daily changes. This decreased uncertainty has implications for facilitating smaller, less-toxic treatment margins, potentially allowing delivery of higher dose radiotherapy that will lead to better control of tumors. The technology has already found success in treating breast, prostate, pancreatic, liver, lung, and limited metastatic cancers, in addition to non-oncologic indications such as cardiac ablation.
1. Introduction
Over the last twenty years, advances in the delivery of conformal radiotherapy, namely through the widespread adoption of intensity-modulated radiotherapy (IMRT) and stereotactic body radiation therapy (SBRT), have facilitated improvements in both tumor control and toxicity. For such conformal radiotherapy to be feasible, it has necessitated advances in image-guided radiotherapy (IGRT), which can help ensure the radiation target volumes are treated while adjacent organs-at-risk (OARs) are spared.
Magnetic resonance imaging (MRI) is an imaging modality that has proven advantages for radiation treatment by aiding in delineating relevant structures since it offers superior soft tissue contrast. Previously, the incorporation of MRI into IGRT technologies has been prohibited due to the magnetic field impacting the shape of the radiation beam via the electron return effect, as well as electronic components of the linear accelerator deteriorating the quality of the MR imaging
[1]. However, recent advances in technology have been able to overcome that challenge and have enabled the development of integrated MR-guided radiotherapy (MRgRT) systems
[2].
A number of MRgRT systems have been developed, including the MRIdian by ViewRay and Unity by Elekta, incorporating both low-field and high-field MRI imaging. These systems are specifically designed to account for the impact of the magnetic field on the trajectory of charged particles (via the Lorenz force) in their dose calculation algorithms
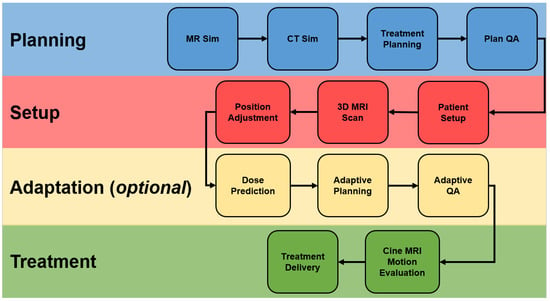
[3]. MRgRT systems offer several advantages compared to other IGRT options including improved soft tissue visualization (which improves target delineation and motion management while enabling dose escalation and adaptive treatments), no radiation exposure (which enables more frequent imaging), real-time monitoring (obviating the need for fiducials), and ability to assess treatment response over the course of treatment. Consequently, tumors with significant motion, tumors with better delineation on MRI, tumors whose treatment benefits from smaller margins, and tumors that benefit from hypofractionation all stand to benefit from MRgRT. The overall clinical workflow of MRgRT is summarized in
Figure 1.
Figure 1. MRgRT. Abbreviations: MR, magnetic resonance; CT, computed tomography; QA, quality assurance; MRI, magnetic resonance imaging.
2. Types of MRgRT
2.1. Low-Field
Low-field MRI-guided radiotherapy uses a magnetic field strength of 0.2 to 1.0 Tesla
[4]. Due to the lower magnetic field strength of low-field, the image resolution is typically lower compared to high-field. A primary example of a low-field MR-linac is the ViewRay MRIdian. Key points regarding low-field MRgRT are summarized in
Table 1.
Table 1.
Comparison of types of MRgRT.
2.2. High Field
High-field MRI-guided radiotherapy uses a magnetic field strength of greater than 1.0 Tesla. A primary example of a high-field MR-linac is the Elekta Unity. Key points regarding high-field MRgRT are summarized in Table 1. Due to the higher image quality, if available, the use of a high-field rather than a low-field MR-linac will generally be preferable, as it will better facilitate the accurate delineation of structures of interest.
2.3. MagnetTx Aurora RT
Distinct from current MR-linac low-field or high-field options is the MagnetTx Aurora RT system that has recently been approved by the Food and Drug Administration. This system consists of a larger 110 × 60 cm bore and an 0.5 T open biplanar magnet
[5]. The configuration allows for configurations where the radiation is either perpendicular or parallel to the MR-linac’s main magnetic field. The parallel configuration is distinct from other systems and limits exit skin dose and dosimetric hotspots associated with the electron return effect and electron streaming effect
[6][7][6,7]. The geometry also creates a more homogeneous dose distribution
[8]. At present, the MagnetTx system has not been used nearly as much as the MRIdian or Unity systems, so future work as more units are installed will further elucidate the clinical benefit of the system.
3. Indications Treated by MRgRT
The way in which MRgRT can improve radiotherapy is dependent on the disease site;
Table 2 summarizes, by disease site, rationales for how MRgRT can benefit clinical management. These disease sites and their clinical evidence are summarized below. The indications summarized below by no means represent a comprehensive list, as MRgRT has potential for most disease sites (including head and neck
[9], colorectal
[10], brain
[11], and gynecological cancers
[12][13][14][12,13,14]) due to similar rationales to the included indications.
Table 2.
Rationale for MRgRT by disease site.
| Disease Site |
Enhanced Soft Tissue Visualization |
Motion Management |
Inter-Fraction Adaptive Re-Planning |
Margin Reduction |
Facilitate Dose Escalation |
Relevant Studies |
| Prostate |
✓ |
|
|
✓ |
|
Kishan et al. [15]
Bruynzeel et al. [16]
Ma et al. [17] |
| Pancreas |
✓ |
✓ |
✓ |
|
✓ |
Rudra et al. [18]
Parikh et al. [19] |
| Liver |
✓ |
✓ |
✓ |
|
✓ |
Rosenberg et al. [20]
Luterstein et al. [21]
van Dams et al. [22]
Ugurluer et al. [23]
Henke et al. [24] |
| Breast |
✓ |
✓ |
|
✓ |
|
Kennedy et al. [25]
Vasmel et al. [26] |
| Lung |
|
✓ |
✓ |
|
|
Henke et al. [27]
Finazzi et al. [28]
Regnery et al. [29]
Finazzi et al. [30]
Finazzi et al. [31] |
| Oligometastases |
✓ |
✓ |
✓ |
✓ |
✓ |
Cuccia et al. [32]
Yoon et al. [33] |
| Cardiac Ablation |
✓ |
✓ |
|
|
|
Mayinger et al. [34] |
4. Future Directions
4.1. Imaging Improvements
A logical step to improve the benefit of MRgRT is to maximize the information made available within the MRI images. Although there is certainly more soft tissue information available and an MRI compared to a CT, MRI is still susceptible to artifacts, particularly during motion. Therefore, finding ways either in acquisition or post-processing to reduce artifacts could overall improve treatment plans. Additionally, different MR imaging sequences have different utilities depending on the clinical scenario. For example, T2 weighted MRI is most useful for delineating cervical cancer GTV, while T1 contrast-enhanced imaging is optimal for many CNS diseases/metastases. Optimizing imaging sequences to provide the most information for target delineation as well as potential treatment response could further enhance the efficacy of MRgRT.
Specific to response assessment, functional imaging is also of interest. Functional imaging is already being incorporated into radiation oncology workflows in the form of biologically guided radiation therapy using integrated positron emission tomography (PET)-based linear accelerators
[35]. MRI functional imaging has potential as well. Diffusion-weighted MRI (DW-MRI) can potentially be correlated with necrosis or apoptosis, and therefore treatment response
[36]. Thus, it might be used to select patients based on early response for treatment escalation or de-escalation where appropriate. In a proof of concept, one study utilized DWI imaging to assess response in patients undergoing neoadjuvant therapy for rectal cancer
[37]. Other imaging sequences are intended to measure the tumor microenvironment such as hypoxia, which can also help tailor radiotherapy by altering the management of tumors that may be more radioresistant
[38]. Lastly, radiomics and quantitative imaging might also be used to monitor treatment response and intervene if needed
[39][40][39,40].
4.2. Adaptive Workflows
MRgRT, and adaptive planning in particular, is associated with additional work compared to traditional non-adaptive CT-based treatment. Future work will need to determine how best to optimize adaptive workflows in order to make routine clinical adoption feasible. The current workflow involves several steps including re-segmentation and, if needed, re-optimization. Given that both adjacent OARs and targets can change on a daily basis, both must be addressed in the adaptive workflow. Existing MRgRT planning systems allow for multiple individuals to work on a single patient at once as part of a parallel adaptive workflow. Therefore, the monitoring of target volumes, OARs, and performance of quality assurance checks can all be performed simultaneously. However, these are still rate-limiting steps and further optimization could lead to new opportunities to speed up the workflow. Options for contouring could include artificial intelligence-based auto-segmentation on a daily basis to provide an improved starting point for contours relative to transferring contours over from the initial simulation. An alternative is to have an off-site team of experts whose job it is to contour relevant structures. Either approach can take the burden off the treating physicians, who may have limited time during the clinic to replan cases on a daily basis.
Another potential for improvement would be to develop decision support tools for deciding when to adapt. In many cases, anatomic changes may not be significant enough to require an adaptation, but the threshold for that decision can be difficult to ascertain without quantitative measures. Automated decision support tools could analyze the initial treatment plan relative to daily MRI setup imaging to provide guidance on which fractions should be adapted and which fractions can be delivered as is.
5. Conclusions
MRgRT represents an exciting and versatile emerging technology that has the potential to significantly improve how radiation can manage both primary and metastatic tumors, including the indications detailed
in this review as well as head and neck, colorectal, brain, and gynecological cancers. As the field of radiotherapy continues to trend towards more hypofractionated high-dose regimens, the importance of MRgRT in improving the therapeutic index to limit major toxicity is significant. Though there is currently significant clinical evidence for the incorporation of MRgRT, future work will seek to demonstrate its superiority over current standard of care options as well as to streamline associated workflows to make MRgRT and adaptive planning a part of routine clinical practice.