The pervasive application of chimeric antigen receptor (CAR)-based cellular therapies in the treatment of oncological diseases has long been recognized. However, CAR T cells can target and eliminate autoreactive cells in autoimmune and immune-mediated diseases. By doing so, they can contribute to an effective and relatively long-lasting remission. In turn, CAR Treg interventions may have a highly effective and durable immunomodulatory effect via a direct or bystander effect, which may have a positive impact on the course and prognosis of autoimmune diseases. CAR-based cellular techniques have a complex theoretical foundation and are difficult to implement in practice, but they have a remarkable capacity to suppress the destructive functions of the immune system.
- chimeric antigen receptor
- CAR T
- CAR Treg
- autoimmune
- immune-mediated
1. CAR T and CAR Treg Therapies and Clinical Trials in Systemic Lupus Erythematosus
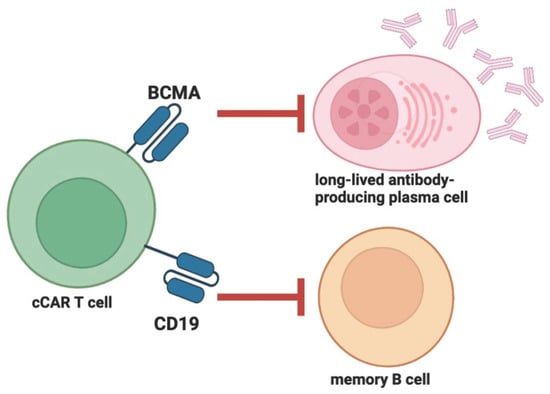
2. Compound CAR T Therapy and Clinical Trials in ANCA-Associated Vasculitis and Autoimmune Hemolytic Anemia
3. CAR T and CAR Treg Therapies in Rheumatoid Arthritis
4. CAR T Therapies and Clinical Trials in Systemic Sclerosis
5. CAR T, CAAR T and CAR Treg Therapies and Clinical Trials in Immune-Mediated Neurological Disorders
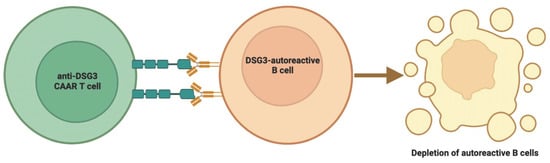
6. CAAR T Therapies and Clinical Trials in Pemphigus Vulgaris
7. CAR T and CAR Treg Therapies and Clinical Trials in Dermatomyositis, Adult-Onset Still’s Disease, and Inflammatory Bowel Disease
8. CAR T Therapy in Type 1 Diabetes Mellitus
9. CAR Treg Therapy and Clinical Trials in Graft-Versus-Host Disease
References
- Radic, M.; Neeli, I.; Marion, T. Prospects for CAR T cell immunotherapy in autoimmune diseases: Clues from Lupus. Expert Opin. Biol. Ther. 2022, 22, 499–507.
- Kansal, R.; Richardson, N.; Neeli, I.; Khawaja, S.; Chamberlain, D.; Ghani, M.; Ghani, Q.-U.; Balazs, L.; Beranova-Giorgianni, S.; Giorgianni, F.; et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci. Transl. Med. 2019, 11, eaav1648.
- Jin, X.; Xu, Q.; Pu, C.; Zhu, K.; Lu, C.; Jiang, Y.; Xiao, L.; Han, Y.; Lu, L. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell. Mol. Immunol. 2021, 18, 1896–1903.
- Mougiakakos, D.; Krönke, G.; Völkl, S.; Kretschmann, S.; Aigner, M.; Kharboutli, S.; Böltz, S.; Manger, B.; Mackensen, A.; Schett, G. CD19-Targeted CAR T Cells in Refractory Systemic Lupus Erythematosus. N. Engl. J. Med. 2021, 385, 567–569.
- Schett, G.; Boeltz, S.; Müller, F.; Kleyer, A.; Völkl, S.; Aigner, M.; Gary, R.; Kretschmann, S.; Simon, D.; Kharboutli, S.; et al. OP0279 CAR-T cell treatment of refractory systemic lupus erythematosus—Safety and preliminary efficacy data from the first four patients. Ann. Rheum. Dis. 2022, 81, 185.
- Mackensen, A.; Müller, F.; Mougiakakos, D.; Böltz, S.; Wilhelm, A.; Aigner, M.; Völkl, S.; Simon, D.; Kleyer, A.; Munoz, L.; et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 2022, 28, 2124–2132.
- Zhang, W.; Feng, J.; Cinquina, A.; Wang, Q.; Xu, H.; Zhang, Q.; Sun, L.; Chen, Q.; Xu, L.; Pinz, K.; et al. Treatment of Systemic Lupus Erythematosus using BCMA-CD19 Compound CAR. Stem Cell Rev. Rep. 2021, 17, 2120–2123.
- Kim, Y.C.; Zhang, A.-H.; Su, Y.; Rieder, S.A.; Rossi, R.J.; Ettinger, R.A.; Pratt, K.P.; Shevach, E.M.; Scott, D.W. Engineered antigen-specific human regulatory T cells: Immunosuppression of FVIII-specific T- and B-cell responses. Blood 2015, 125, 1107–1115.
- Guo, H.; Xun, L.; Zhang, R.; Hu, F.; Luan, J.; Lao, K.; Wang, X.; Gou, X. Stability and inhibitory function of Treg cells under inflammatory conditions in vitro. Exp. Ther. Med. 2020, 18, 2443–2450.
- He, J.; Zhang, R.; Shao, M.; Zhao, X.; Miao, M.; Chen, J.; Liu, J.; Zhang, X.; Zhang, X.; Jin, Y.; et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: A randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2019, 79, 141–149.
- Stone, J.H.; Merkel, P.A.; Spiera, R.; Seo, P.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.; St Clair, E.W.; Turkiewicz, A.; Tchao, N.K.; et al. Faculty Opinions recommendation of Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 2011, 363, 221–232.
- Jones, R.B.; Furuta, S.; Tervaert, J.W.; Hauser, T.; Luqmani, R.; Morgan, M.D.; Peh, C.A.; Savage, C.O.; Segelmark, M.; Tesar, V.; et al. Rituximab versus cyclophosphamide in AN-CA-associated renal vasculitis: 2-year results of a randomised trial. Ann. Rheum. Dis. 2015, 74, 1178–1182.
- Garvey, B. Rituximab in the treatment of autoimmune haematological disorders. Br. J. Haematol. 2008, 141, 149–169.
- Rodrigo, C.; Rajapakse, S.; Gooneratne, L. Rituximab in the treatment of autoimmune haemolytic anaemia. Br. J. Clin. Pharmacol. 2015, 79, 709–719.
- Shen, Q.; Du, Y. A comprehensive review of advanced drug delivery systems for the treatment of rheumatoid arthritis. Int. J. Pharm. 2023, 635, 122698.
- Harre, U.; Georgess, D.; Bang, H.; Bozec, A.; Axmann, R.; Ossipova, E.; Jakobsson, P.-J.; Baum, W.; Nimmerjahn, F.; Szarka, E.; et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Investig. 2012, 122, 1791–1802.
- Vossenaar, E.R.; Després, N.; Lapointe, E.; van der Heijden, A.; Lora, M.; Senshu, T.; van Venrooij, W.J.; Ménard, H.A. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res. Ther. 2004, 6, R142–R150.
- Zanetakis, E.; Rigby, W.F.; Rubbert-Roth, A.; Peterfy, C.G.; Van Vollenhoven, R.F.; Stohl, W.; Hessey, E.; Chen, A.; Tyrrell, H.; Hackshaw, K.; et al. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: The IMAGE trial. Ann. Rheum. Dis. 2011, 70, 39–46.
- Zhang, B.; Wang, Y.; Yuan, Y.; Sun, J.; Liu, L.; Huang, D.; Hu, J.; Wang, M.; Li, S.; Song, W.; et al. In vitro elimination of autoreactive B cells from rheumatoid arthritis patients by universal chimeric antigen receptor T cells. Ann. Rheum. Dis. 2021, 80, 176–184.
- Li, Y.-J.; Chen, Z. Cell-based therapies for rheumatoid arthritis: Opportunities and challenges. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221100294.
- Van Steendam, K.; Tilleman, K.; De Ceuleneer, M.; De Keyser, F.; Elewaut, D.; Deforce, D. Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins. Arthritis Res. Ther. 2010, 12, R132.
- Orvain, C.; Boulch, M.; Bousso, P.; Allanore, Y.; Avouac, J. Is There a Place for Chimeric Antigen Receptor-T Cells in the Treatment of Chronic Autoimmune Rheumatic Diseases? Arthritis Rheumatol. 2021, 73, 1954–1965.
- Yoshizaki, A. Pathogenic roles of B lymphocytes in systemic sclerosis. Immunol. Lett. 2018, 195, 76–82.
- Pillai, S. T and B lymphocytes in fibrosis and systemic sclerosis. Curr. Opin. Rheumatol. 2019, 31, 576–581.
- Brown, M.; O’Reilly, S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin. Exp. Immunol. 2019, 195, 310–321.
- Jordan, S.; Distler, J.H.; Maurer, B.; Huscher, D.; van Laar, J.M.; Allanore, Y.; Distler, O.; EUSTAR Rituximab study group. Effects and safety of rituximab in systemic sclerosis: An analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann. Rheum. Dis. 2015, 74, 1188–1194.
- Daoussis, D.; Melissaropoulos, K.; Sakellaropoulos, G.; Antonopoulos, I.; Markatseli, T.E.; Simopoulou, T.; Georgiou, P.; Andonopoulos, A.P.; Drosos, A.A.; Sakkas, L.; et al. A multicenter, open-label, comparative study of B-cell depletion therapy with Rituximab for systemic sclerosis-associated interstitial lung disease. Semin. Arthritis Rheum. 2017, 46, 625–631.
- Kuzumi, A.; Ebata, S.; Fukasawa, T.; Matsuda, K.M.; Kotani, H.; Yoshizaki-Ogawa, A.; Sato, S.; Yoshizaki, A. Long-term Outcomes After Rituximab Treatment for Patients With Systemic Sclerosis: Follow-up of the DESIRES Trial With a Focus on Serum Immunoglobulin Levels. JAMA Dermatol. 2023, 159, e226340.
- Aghajanian, H.; Kimura, T.; Rurik, J.G.; Hancock, A.S.; Leibowitz, M.S.; Li, L.; Scholler, J.; Monslow, J.; Lo, A.; Han, W.; et al. Targeting cardiac fibrosis with engineered T cells. Nature 2019, 573, 430–433.
- Rurik, J.G.; Tombácz, I.; Yadegari, A.; Fernández, P.O.M.; Shewale, S.V.; Li, L.; Kimura, T.; Soliman, O.Y.; Papp, T.E.; Tam, Y.K.; et al. CAR T cells produced in vivo to treat cardiac injury. Science 2022, 375, 91–96.
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Faculty Opinions recommendation of Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 221–234.
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; et al. Faculty Opinions recommendation of Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med. 2016, 376, 209–220.
- Mitsdoerffer, M.; Di Liberto, G.; Dötsch, S.; Sie, C.; Wagner, I.; Pfaller, M.; Kreutzfeldt, M.; Fräßle, S.; Aly, L.; Knier, B.; et al. Formation and immunomodulatory function of meningeal B cell aggregates in progressive CNS autoimmunity. Brain 2021, 144, 1697–1710.
- Weber, M.S.; Prod’homme, T.; Patarroyo, J.C.; Molnarfi, N.; Karnezis, T.; Lehmann-Horn, K.; Danilenko, D.M.; Eastham-Anderson, J.; Slavin, A.J.; Linington, C.; et al. B-cell activation influences T-cell polarization and outcome of an-ti-CD20 B-cell depletion in central nervous system autoimmunity. Ann. Neurol. 2010, 68, 369–383.
- Häusler, D.; Häusser-Kinzel, S.; Feldmann, L.; Torke, S.; Lepennetier, G.; Bernard, C.C.A.; Zamvil, S.S.; Brück, W.; Lehmann-Horn, K.; Weber, M.S. Functional characterization of reappearing B cells after anti-CD20 treatment of CNS autoimmune disease. Proc. Natl. Acad. Sci. USA 2018, 115, 9773–9778.
- Gupta, S.; Simic, M.; Sagan, S.A.; Shepherd, C.; Duecker, J.; Sobel, R.A.; Dandekar, R.; Wu, G.F.; Wu, W.; Pak, J.E.; et al. CAR-T Cell–Mediated B-Cell Depletion in Central Nervous System Autoimmunity. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200080.
- Monson, N.L.; Cravens, P.D.; Frohman, E.M.; Hawker, K.; Racke, M.K. Effect of Rituximab on the Peripheral Blood and Cerebrospinal Fluid B Cells in Patients With Primary Progressive Multiple Sclerosis. Arch. Neurol. 2005, 62, 258–264.
- Mainero, C.; Louapre, C. Meningeal inflammation in multiple sclerosis: The key to the origin of cortical lesions? Neurology 2015, 85, 12–13.
- Abramson, J.S.; McGree, B.; Noyes, S.; Plummer, S.; Wong, C.; Chen, Y.-B.; Palmer, E.; Albertson, T.; Ferry, J.A.; Arrillaga-Romany, I.C. Anti-CD19 CAR T Cells in CNS Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 783–784.
- Fransson, M.; Piras, E.; Burman, J.; Nilsson, B.; Essand, M.; Lu, B.; Harris, R.A.; Magnusson, P.U.; Brittebo, E.; Loskog, A.S. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J. Neuroinflamm. 2012, 9, 112.
- Kim, Y.C.; Zhang, A.H.; Yoon, J.; Culp, W.E.; Lees, J.R.; Wucherpfennig, K.W.; Scott, D.W. Engineered MBP-specific human Tregs ameliorate MOG-induced EAE through IL-2-triggered inhibition of effector T cells. J. Autoimmun. 2018, 92, 77–86.
- De Paula Pohl, A.; Schmidt, A.; Zhang, A.H.; Maldonado, T.; Königs, C.; Scott, D.W. Engineered regulatory T cells expressing myelin-specific chimeric antigen receptors suppress EAE progression. Cell Immunol. 2020, 358, 104222.
- Feinberg, M.B.; McCune, J.M.; Miedema, F.; Moore, J.P.; Schuitemaker, H. HIV tropism and CD4+ T-cell depletion. Nat. Med. 2002, 8, 537.
- Ghaleh, H.E.G.; Bolandian, M.; Dorostkar, R.; Jafari, A.; Pour, M.F. Concise review on optimized methods in production and transduction of lentiviral vectors in order to facilitate immunotherapy and gene therapy. Biomed. Pharmacother. 2020, 128, 110276.
- Sedaghat, N.; Etemadifar, M. Inducing chimeric antigen receptor (CAR) regulatory T cells in-vivo: A novel concept for a potential feasible cure of demyelinating diseases. Mult. Scler. Relat. Disord. 2021, 57, 103341.
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia gravis. Nat. Rev. Dis. Prim. 2019, 5, 30.
- Lazaridis, K.; Tzartos, S.J. Myasthenia Gravis: Autoantibody Specificities and Their Role in MG Management. Front. Neurol. 2020, 11, 596981.
- Behin, A.; Le Panse, R. New Pathways and Therapeutic Targets in Autoimmune Myasthenia Gravis. J. Neuromuscul. Dis. 2018, 5, 265–277.
- Bagherieh, S.; Afshari-Safavi, A.; Vaheb, S.; Kiani, M.; Ghaffary, E.M.; Barzegar, M.; Shaygannejad, V.; Zabeti, A.; Mirmosayyeb, O. Worldwide prevalence of neuromyelitis optica spectrum disorder (NMOSD) and neuromyelitis optica (NMO): A systematic review and meta-analysis. Neurol. Sci. 2023, 44, 1905–1915.
- Wolbert, J.; Cheng, M.; Zu Horste, G.M.; Su, M.A. Deciphering immune mechanisms in chronic inflammatory demyelinating polyneuropathies. J. Clin. Investig. 2020, 5, e132411.
- Bright, R.J.; Wilkinson, J.; Coventry, B.J. Therapeutic options for chronic inflammatory demyelinating polyradiculoneuropathy: A systematic review. BMC Neurol. 2014, 14, 26.
- Stuhlmüller, B.; Schneider, U.; González-González, J.B.; Feist, E. Disease specific autoantibodies in idiopathic inflammatory myopathies. Front. Neurol. 2019, 10, 438.
- Xiong, A.; Yang, G.; Song, Z.; Xiong, C.; Liu, D.; Shuai, Y.; He, L.; Zhang, L.; Guo, Z.; Shuai, S. Rituximab in the treatment of immune-mediated necrotizing myopathy: A review of case reports and case series. Ther. Adv. Neurol. Disord. 2021, 14, 1756286421998918.
- Zhao, L.; Chen, Y.; Wang, M.; Leyang, X. The global incidence rate of pemphigus vulgaris: A systematic review and meta-analysis. Dermatology 2023, 239, 1–9.
- Ellebrecht, C.T.; Bhoj, V.G.; Nace, A.; Choi, E.J.; Mao, X.; Cho, M.J.; Di Zenzo, G.; Lanzavecchia, A.; Seykora, J.T.; Cotsarelis, G.; et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 2016, 353, 179–184.
- Parvathaneni, K.; Scott, D.W. Engineered FVIII-expressing cytotoxic T cells target and kill FVIII-specific B cells in vitro and in vivo. Blood Adv. 2018, 2, 2332–2340.
- Ohyama, B.; Nishifuji, K.; Chan, P.T.; Kawaguchi, A.; Yamashita, T.; Ishii, N.; Hamada, T.; Dainichi, T.; Koga, H.; Tsuruta, D.; et al. Epitope Spreading Is Rarely Found in Pemphigus Vulgaris by Large-Scale Longitudinal Study Using Desmoglein 2–Based Swapped Molecules. J. Investig. Dermatol. 2012, 132, 1158–1168.
- Lee, J.; Lundgren, D.K.; Mao, X.; Manfredo-Vieira, S.; Nunez-Cruz, S.; Williams, E.F.; Assenmacher, C.-A.; Radaelli, E.; Oh, S.; Wang, B.; et al. Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris. J. Clin. Investig. 2020, 130, 6317–6324.
- Schmidt, E.; Spindler, V.; Eming, R.; Amagai, M.; Antonicelli, F.; Baines, J.F.; Belheouane, M.; Bernard, P.; Borradori, L.; Caproni, M.; et al. Meeting Report of the Pathogenesis of Pemphigus and Pemphigoid Meeting in Munich, September 2016. J. Investig. Dermatol. 2017, 137, 1199–1203.
- Siddiqi, H.F.; Staser, K.W.; Nambudiri, V.E. Research Techniques Made Simple: CAR T-Cell Therapy. J. Investig. Dermatol. 2018, 138, 2501–2504.e1.
- Colliou, N.; Picard, D.; Caillot, F.; Calbo, S.; Le Corre, S.; Lim, A.; Lemercier, B.; Le Mauff, B.; Maho-Vaillant, M.; Jacquot, S.; et al. Long-Term Remissions of Severe Pemphigus After Rituximab Therapy Are Associated with Prolonged Failure of Desmoglein B Cell Response. Sci. Transl. Med. 2013, 5, 175ra30.
- Amagai, M. Pemphigus vulgaris and its active disease mouse model. Curr. Dir. Autoimmun. 2008, 10, 167–181.
- Nishifuji, K.; Amagai, M.; Kuwana, M.; Iwasaki, T.; Nishikawa, T. Detection of Antigen-Specific B Cells in Patients with Pemphigus Vulgaris by Enzyme-Linked Immunospot Assay: Requirement of T Cell Collaboration for Autoantibody Production. J. Investig. Dermatol. 2000, 114, 88–94.
- Basu, S.; Volkov, J.; Nunez, D.; Fouch, M.; Stadanlick, J.; Binder, G.; Chang, D.; Hoffman, K.; Porter, D.; Abedi, M.; et al. Characterization of DSG3-CAART cells prior to & following adoptive transfer in mucosal Pemphigus Vulgaris. Hum. Gene Ther. 2022, 33, A123.
- Mueller, K.T.; Maude, S.L.; Porter, D.L.; Frey, N.; Wood, P.; Han, X.; Waldron, E.; Chakraborty, A.; Awasthi, R.; Levine, B.L.; et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 2017, 130, 2317–2325.
- Tomaras, S.; Feist, E. Myositissyndrome . Inn. Med. 2023, 64, 152–163.
- Mavragani, C.P.; Spyridakis, E.G.; Koutsilieris, M. Adult-Onset Still’s Disease: From Pathophysiology to Targeted Therapies. Int. J. Inflamm. 2012, 2012, 879020.
- Otte, M.L.; Tamang, R.L.; Papapanagiotou, J.; Ahmad, R.; Dhawan, P.; Singh, A.B. Mucosal healing and inflammatory bowel disease: Therapeutic implications and new targets. World J. Gastroenterol. 2023, 29, 1157–1172.
- Gordon, H.; Rodger, B.; Lindsay, J.O.; Stagg, A.J. Recruitment and residence of intestinal T cells—Lessons for therapy in IBD. J. Crohn’s Colitis 2023, 17, jjad027.
- Radziszewska, A.; Moulder, Z.; Jury, E.C.; Ciurtin, C. CD8+ T Cell Phenotype and Function in Childhood and Adult-Onset Connective Tissue Disease. Int. J. Mol. Sci. 2022, 23, 11431.
- Jung, J.-Y.; Choi, B.; Sayeed, H.; Suh, C.-H.; Kim, Y.W.; Kim, H.-A.; Sohn, S. Characteristic patterns of HLA presentation and T cell differentiation in adult-onset Still’s disease. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418791284.
- Dai, Z.; Mu, W.; Zhao, Y.; Cheng, J.; Lin, H.; Ouyang, K.; Jia, X.; Liu, J.; Wei, Q.; Wang, M.; et al. T cells expressing CD5/CD7 bispecific chimeric antigen receptors with fully human heavy-chain-only domains mitigate tumor antigen escape. Signal Transduct. Target. Ther. 2022, 7, 85.
- Pan, J.; Tan, Y.; Wang, G.; Deng, B.; Ling, Z.; Song, W.; Seery, S.; Zhang, Y.; Peng, S.; Xu, J.; et al. Donor-Derived CD7 Chimeric Antigen Receptor T Cells for T-Cell Acute Lymphoblastic Leukemia: First-in-Human, Phase I Trial. J. Clin. Oncol. 2021, 39, 3340–3351.
- Breman, E.; Demoulin, B.; Agaugué, S.; Mauën, S.; Michaux, A.; Springuel, L.; Houssa, J.; Huberty, F.; Jacques-Hespel, C.; Marchand, C.; et al. Overcoming Target Driven Fratricide for T Cell Therapy. Front. Immunol. 2018, 9, 2940.
- Moll, M.; Reinhold, U.; Kukel, S.; Abken, H.; Müller, R.; Oltermann, I.; Kreysel, H.W. CD7-negative helper T cells accumulate in inflammatory skin lesions. J. Investig. Dermatol. 1994, 102, 328–332.
- Schmidt, D.; Goronzy, J.J.; Weyand, C.M. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J. Clin. Investig. 1996, 97, 2027–2037.
- Bishu, S.; Arsenescu, V.; Lee, E.Y.; Vargas, H.D.; De Villiers, W.J.; Arsenescu, R. Autoimmune enteropathy with a CD8+ CD7-T-cell small bowel intraepithelial lymphocytosis: Case report and literature review. BMC Gastroenterol. 2011, 11, 131.
- Bao, L.; Bo, X.-C.; Cao, H.-W.; Qian, C.; Wang, Z.; Li, B. Engineered T cells and their therapeutic applications in autoimmune diseases. Zool. Res. 2022, 43, 150–165.
- Elinav, E.; Adam, N.; Waks, T.; Eshhar, Z. Amelioration of Colitis by Genetically Engineered Murine Regulatory T Cells Redirected by Antigen-Specific Chimeric Receptor. Gastroenterology 2009, 136, 1721–1731.
- Elinav, E.; Waks, T.; Eshhar, Z. Redirection of Regulatory T Cells With Predetermined Specificity for the Treatment of Experimental Colitis in Mice. Gastroenterology 2008, 134, 2014–2024.
- McGovern, J.L.; Wright, G.P.; Stauss, H.J. Engineering Specificity and Function of Therapeutic Regulatory T Cells. Front. Immunol. 2017, 8, 1517.
- Freen-van Heeren, J.J. Using CRISPR to enhance T cell effector function for therapeutic applications. Cytokine X 2020, 3, 100049.
- Blat, D.; Zigmond, E.; Alteber, Z.; Waks, T.; Eshhar, Z. Suppression of Murine Colitis and its Associated Cancer by Carcinoembryonic Antigen-Specific Regulatory T Cells. Mol. Ther. 2014, 22, 1018–1028.
- Zmievskaya, E.; Valiullina, A.; Ganeeva, I.; Petukhov, A.; Rizvanov, A.; Bulatov, E. Application of CAR-T Cell Therapy beyond Oncology: Autoimmune Diseases and Viral Infections. Biomedicines 2021, 9, 59.
- Syed, F.Z. Type 1 Diabetes Mellitus. Ann. Intern. Med. 2022, 175, ITC33–ITC48.
- Fishman, S.; Lewis, M.D.; Siew, L.K.; De Leenheer, E.; Kakabadse, D.; Davies, J.; Ziv, D.; Margalit, A.; Karin, N.; Gross, G.; et al. Adoptive Transfer of mRNA-Transfected T Cells Redirected against Diabetogenic CD8 T Cells Can Prevent Diabetes. Mol. Ther. 2017, 25, 456–464.
- Zhang, L.; Sosinowski, T.; Cox, A.R.; Cepeda, J.R.; Sekhar, N.S.; Hartig, S.M.; Miao, D.; Yu, L.; Pietropaolo, M.; Davidson, H.W. Chimeric antigen receptor (CAR) T cells targeting a pathogenic MHC class II: Peptide complex modulate the progression of autoimmune diabetes. J. Autoimmun. 2019, 96, 50–58.
- Tenspolde, M.; Zimmermann, K.; Weber, L.C.; Hapke, M.; Lieber, M.; Dywicki, J.; Frenzel, A.; Hust, M.; Galla, M.; Buitrago-Molina, L.E.; et al. Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. J. Autoimmun. 2019, 103, 102289.
- Sobkowiak-Sobierajska, A.; Lindemans, C.; Sykora, T.; Wachowiak, J.; Dalle, J.H.; Bonig, H.; Gennery, A.; Lawitschka, A. Management of Chronic Graft-vs.-Host Disease in Children and Adolescents With ALL: Present Status and Model for a Personalised Management Plan. Front. Pediatr. 2022, 10, 808103.
- Imura, Y.; Ando, M.; Kondo, T.; Ito, M.; Yoshimura, A. CD19-targeted CAR regulatory T cells suppress B cell pathology without GvHD. J. Clin. Investig. 2020, 5, e136185.
