1. Introduction
1.1. Epidemiology and Risk Factors for Breast Cancer
Cancer is a major public health problem worldwide, as the World Health Organization (WHO) estimates that by 2040, there will be 28.9 million new cases that will cause more than 16.2 million deaths annually. Although breast cancer mainly affects women, it has become the most diagnosed malignancy in the general population, precisely because of the number of cases diagnosed in women, surpassing lung cancer, causing more than 2.2 million new cases (11.7%) and more than 680,000 deaths in 2020 alone, corresponding to one in six cancer deaths in women
[1]. The burden of breast cancer is expected to increase year after year, despite having a high remission rate once the disease is identified and treated prior to progression to metastatic disease
[2][3][4][2,3,4].
The risk factors associated with this disease include both intrinsic and extrinsic factors. The intrinsic factors are not avoidable and are associated with genetic and epigenetic characteristics
[5], including mutations in autosomal dominant genes, such as breast cancer 1 (
BRCA1) and breast cancer 2 (
BRCA2)
[6]; mutations in moderate-risk genes, such as the CHK2 serine/threonine protein kinase gene (
CHEK2), the ataxia telangiectasia gene (
ATM), and the partner and localize of BRCA2 gene (
PALB2); or low-frequency variations, such as single-nucleotide polymorphisms (SNPs). The extrinsic factors are avoidable factors, such as sedentary lifestyles, obesity, alcohol, tobacco or drug use, use of birth control pills or hormone replacement therapies, and breast density
[7][8][7,8]. In addition, it was observed that parity and age at menarche are implicated in the risk of breast cancer
[9][10][9,10] and that different sociodemographic characteristics, such as lack of education, presence of anxiety or depression, or above-average comorbidities, cause a delay in the treatment of patients
[11].
1.2. Heterogeneity of Breast Cancer: Progression of the Disease and Histological and Molecular Classifications
Generally, tumors develop following a sequence of initial lesions or alterations, hyperplasia, dysplasia, carcinoma in situ and invasive carcinoma. Traditionally, the histological classification scheme for breast cancer has been divided into (1) carcinoma in situ, which comprise noninvasive tumors with potentially malignant intraductal cells confined to the ducts (ductal carcinoma in situ) or lobules (lobular carcinoma in situ) from which cells can evolve uncontrollably to invasive, or (2) infiltrative carcinoma, in which neoplastic cells have penetrated stroma
[12]. Although current consensus recognizes invasive ductal and lobular carcinomas, it was reported that most of these tumors arise in terminal ductal–lobular units (TDLUs) regardless of the histologic type
[13]. Ductal carcinoma is the most commonly diagnosed invasive breast cancer, accounting for 50–75% of cases, followed by lobular carcinoma (5–15%) and mixed ductal/lobular carcinomas
[14].
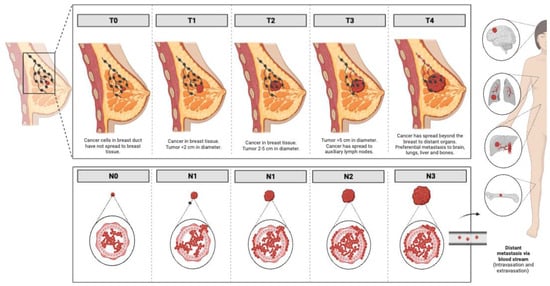
As for the progression of the disease, the traditional TNM staging system, which is based on tumor (T) anatomic features, regional lymph nodes (N) involvement, and the presence or absence of metastases (M) (
Figure 1), has been the gold standard for determining patient prognosis over the last 70 years
[15]. Although it was reported that breast cancers diagnosed at stages I and II have an overall survival of over 95%, up to 72% when diagnosed at stage III and reduced to 22% when diagnosed at stage IV
[16], this anatomically based system is not enough to address the tumor biology and guide decision-making and treatment planning for all breast cancers, e.g., the triple-negative subtype is difficult to manage
[17]. The eighth edition of the American Joint Committee on Cancer, announced in 2017 and globally adopted on 1 January 2018, also integrated biomarkers such as tumor grade, hormone receptor status, expression of the human epidermal growth factor receptor (EGFR) family member HER2 (Human Epidermal Growth Factor Receptor 2/ErbB2 receptor tyrosine kinase 2) or multigene panel status for certain sub-groups, resulting in different prognostic stages for tumors with virtually identical histologic types
[18]. These have highlighted the important role of breast cancer heterogeneity in the correct clinical management of the disease.
Figure 1.
Progression of breast cancer in the different stages according to the traditional TNM staging system. Images were created using
.
With respect to hormone receptors (estrogen receptor (ER∝) and progesterone receptor (PR)) and human epidermal growth factor receptor 2 (HER2):
-
Hormone-receptor-positive breast tumors, which account for 75% of breast carcinomas, are classified into luminal A breast tumors (50–60% of diagnosed cases)—which are ER-positive and/or PR-positive, HER2-negative and Ki67 < 14%
[19] with low histological grade, and have a low mitosis proportion number and good prognosis—and luminal B tumors (15–20% of diagnosed cases)—which are defined as ER-positive and/or PR-positive (PR < 20% + Ki67 ≥ 14%), HER2-negative or ER-positive and/or PR positive/negative (any PR-positive and any Ki67) and HER2-positive. Luminal B tumors usually have a more aggressive phenotype, both by histologic grade and proliferative Ki67 index, and worse prognosis than luminal A tumors
[20].
-
HER2-enriched tumors, which account for approximately 15–20% of breast tumors, present HER2 overexpression
[21]. These tumors do not express estrogen or progesterone receptors and are characterized by the overactivation of signaling pathways involved in increased cell proliferation (Ras/MAPK mitogen-activated protein kinases and PI3K/AKT phosphoinositide 4-kinase/protein kinase B), with increased risk of metastasis and a more aggressive phenotype than luminal tumors
[22].
-
Basal-like tumors are characterized by a lack of HER2 overexpression and the absence or low levels of ER/PR expression. Among basal-like tumors, the triple-negative subtype, which constitutes approximately 80% of basal-like tumors and 10–15% of breast carcinomas, is defined by the lack of hormone receptors (ER-, PR-), the lack of HER overexpression (HER2-) and being cytokeratin-5/6-positive (CK5/6+) and/or Epidermal-Growth-Factor-Receptor-positive (EGFR+)
[23].
The molecular classification of breast cancer has allowed for the development of personalized therapeutic options, which have greatly improved patient response and prognosis. Since estrogen receptors are steroid hormone receptors that induce the production of growth factors, such as Epidermal Growth Factor (EGF), Insulin-like Growth Factor-1 (IGF) or Transforming growth factor alpha (TGFα), which stimulate tumor cell proliferation, competitive estrogen–estrogen receptor inhibitors have shown their utility to decrease tumor cell proliferation
[24][25][26][24,25,26]. In such a manner, targeted treatments based on the use of monoclonal antibodies revolutionized the treatment for HER2-enriched breast tumors
[27]. Unfortunately, although TNBCs are associated with poor long-term prognosis, higher probabilities of recurrence over time, and high probabilities of local and distant recurrence
[28][29][28,29], no effective therapy has yet been approved for the targeted treatment of these tumors. The 5-year overall survival for non-metastatic disease is 85% for TNBC stage I patients compared with 94–99% for stage I patients with hormone-receptor-positive and HER2-positive breast tumors
[25]. However, the overall 5-year survival rate for patients with metastatic disease is 22%
[30].
These molecular classifications with major predictive and prognostic implications opened the way to histologic-independent personalized therapies, such as poly-ADP ribose polymerase (PARP) inhibitors for the treatment of tumors with mutations in
BRCA1 and
BRCA2 genes (present in up to 5% of breast cancer patients
[31]) by preventing tumor cells with BRCA1/BRCA2 mutations from repairing DNA damage caused by cytotoxic chemotherapy
[32].
As for more advanced transcriptomic analyses, i.e., assays that analyze the expression of multiple genes with the aim of providing prognostic and predictive information about breast cancer patients, these should be used at the time of initial diagnosis, not after relapse, and help to make therapeutic decisions when this is not clearly based on traditional clinicopathologic features
[33][34][33,34]. Oncotype DX is a test developed by the Genomic Health, Inc., laboratory that analyzes 16 cancer-related genes for the diagnosis and prediction of ER-positive and HER2-negative cancer patients. MammaPrint analyzes the expression of 80 genes that allow the tumor to be categorized as luminal A, luminal B or basal-like
[34]. Prosigna is a test designed for HER-positive postmenopausal patients and analyzes the expression of 50 genes to categorize the tumor into luminal A, luminal B, basal-like or HER2-enriched subtypes
[33].
1.3. Conventional Treatments for Breast Cancer
The conventional treatments used to treat breast cancer are surgery, radiotherapy, chemotherapy, hormone therapy and immunotherapy, used alone or in combination.
Breast cancer surgery usually consists of two options: conservative surgery and mastectomy. Currently, breast-conserving surgery has replaced mastectomy, as the overall and disease-free survival rates are equivalent to this radical procedure. In addition, current early diagnosis programs have made the early detection of tumors possible, which allows for avoiding radical mastectomies in most cases. Although with conservative surgery, only the tumor mass is removed, sometimes it is necessary to remove more than 20% of the normal breast tissue surrounding the tumor, which has implications for the physical, emotional, and mental health of the patient. In recent years, the implementation of neoadjuvant chemotherapy has allowed for reducing the tumor size before surgery and, therefore, more conservative surgical interventions. Moreover, the incorporation of sentinel lymph node biopsy into surgery has made it possible to reduce the extent of surgery without compromising the prognostic value
[35][36][37][35,36,37] since patients with one or two positive sentinel nodes should no longer undergo axillary lymphadenectomy
[38].
Radiation therapy by means of X-rays or gamma rays is usually used to eliminate possible cancer cells that remain in the area after surgery. This additional component of breast-conserving therapy, which includes strong enough radiation doses that ensure the complete elimination of malignant cells
[39], can be omitted in patients with limited life expectancy, adjuvant endocrine therapy, negative nodes, and hormone-receptor-positive or HER2-negative tumors
[37].
Chemotherapy can be applied both before surgery to reduce tumor size (neoadjuvant chemotherapy) and avoid mastectomy and/or after surgery (adjuvant chemotherapy), while always considering the tumor size, hormone and HER2 receptor status, as well as the lymph node status
[40]. Adjuvant chemotherapy is usually recommended for patients with a high risk of disease recurrence and usually involves combined treatment with taxanes and anthracyclines. For patients at low risk, anthracyclines are usually omitted
[41].
Hormone therapy is the first-line option for all patients with ER-expressing breast cancer, where tamoxifen (Nolvadex, AstraZeneca Pharmaceutics, Cambridge, UK), which is a selective receptor estrogen modulator, is the drug commonly used because of its ability to reduce disease recurrence by half
[42]. In postmenopausal women, tamoxifen is replaced by aromatase inhibitor drugs, which also target the estrogen signaling pathway, such as anastrozole (Arimidex, AstraZeneca Pharmaceutics, United Kingdom) or letrozole (Femara, Novartis Pharma, Basel, Switzerland)
[43], as they produce a greater reduction in breast cancer recurrence than tamoxifen alone
[44]. However, the best way to use these therapies is still uncertain
[45].
The use of monoclonal antibodies opened a new era in the fight against breast with targeted treatments (
Table 1). HER2, which plays a key role in tumor growth by activating different signaling pathways closely linked to cell proliferation, can be targeted with Trastuzumab (Herceptin, Roche Registration GmbH, Grenzach-Wyhlen, Germany) and pertuzumab (Perjeta, Roche Registration GmbH, Germany), which are two monoclonal antibodies that inhibit HER2 through the extracellular domain of the receptor
[46], thereby blocking the signaling pathways it controls, and thus, exerting a considerable antitumor effect
[47]. In 1998, Trastuzumab became the first monoclonal antibody approved by the FDA to treat HER2-positive breast cancer patients. Pertuzumab was approved in 2013 by the FDA for use in combination with Trastuzumab for HER2-positive patients at risk of relapse
[48], which is a scheme that has shown good tolerability and a decrease in associated side effects
[49]. In 2021, the FDA approved Margetuximab (Margenza, Macrogenics, Rockville, MD, USA) as a monoclonal antibody against HER2 for patients with HER2-positive metastatic breast cancer
[50][51][50,51], the use of which in combination with chemotherapy significantly improves overall survival, although with important associated adverse effects
[52].
Table 1.
Types of immunotherapeutics used to treat breast cancer.
Mutations that cause errors in the DNA replication process, as well as those affecting the DNA repair machinery, are common in the development of cancer
[79]. Poly ADP-ribose polymerase (PARP) enzymes, which are involved in DNA repair, and members of the BER pathway, which is the base excision repair pathway, are critical
[80]. In this regard, olaparib (Lynparza, AstraZeneca AB, Södertälje, Sweden) was the first drug approved by the FDA in 2018 for the treatment of patients with HER2-negative breast cancer and BRCA mutations
[81], but it has many reported side effects
[82]. Talazoparib (Talzenna, Pfizer Europe MA EEIG, Belgium) is another drug approved by the FDA in 2018, but for HER2-negative and locally advanced or BRCA-mutated patients. In vitro, it showed 200-fold greater antitumor results than other PARP inhibitors
[83]. However, the list of associated side effects is equally extensive
[84].
Table 2 shows the main targeted therapies for treating breast cancer employed today.
Table 2.
Types of targeted therapies against breast cancer.
Other HER2-associated monoclonal antibodies include epidermal growth factor receptor (EGFR) and transforming growth factor alpha (TFGα), whose binding activates the PTEN/I3K/Akt/mTOR and Ras/Raf/MEK intracellular signaling pathways. These are directly involved in cell proliferation and apoptosis. Inhibitors of kinases (TKIs) inhibitors at the extracellular domain level of HER2, such as lapatinib (Tyverb, Novartis Europharm Limited, Dublin, Ireland), neratinib (Nerlynx, Pierre Fabre Medicament, Paris, France), tucatinib (Tukysa, Seagen B.V., Schiphol, The Netherlands) and pyrotinib (AiRuiNi, Jiangsu Hengrui Pharmaceutical Group Co., Ltd., Lianyungang, China), are noteworthy in this regard
[53]. Lapatinib is a HER2 and EGFR tyrosine kinase inhibitor at the kinase ATP binding site level and was approved in 2018 by the FDA for HER2-positive patients in combination with other anti-HER2 agents, such as trastuzumab
[54]. Neratinib binds to the tyrosine kinase domain of HER2 and was approved by the FDA in 2018 likewise for HER2-positive stage I to III patients who received adjuvant therapy with trastuzumab
[55]. Tucatinib, which is highly selective against HER2, was approved in 2020 by the FDA for advanced-stage HER2-positive patients and in combination with trastuzumab
[56]. Finally, pyrotinib is a HER inhibitor that was approved in 2018 in China for advanced-stage HER2-positive patients who received prior chemotherapy
[57].
The recent incorporation of drug–antibody conjugates against breast cancer represent an innovative therapeutic approach that combines the high specificity and antitumoral properties of monoclonal antibodies with the potent cytotoxic activity of small molecule drugs
[58]. Examples of these conjugates are (1) trastuzumab emtansine (T-DM1) (Kadcyla, Roche Pharma AG, Germany), which includes trastuzumab, and a maitansinoid deriv-ative, which depolymerizes cell microtubules and triggers cell apoptosis
[59], were ap-proved by the FDA in 2013 for patients with HER2-positive metastatic breast cancer
[60], and (2) Trastuzumab deruxtecan (DS-8201a) (Enhertu, Daiichi Sankyo Europe GmbH, Munich, Germany), which was approved by the FDA in 2020 for the treatment of HER2-positive breast cancers and is composed of trastuzumab, a maleimide, and a topoisomerase inhibitor
[58][61][58,61].
1.4. Targeted Therapies for Breast Cancer
Angiogenesis is a process directly involved in tumor development, as tumor formation depends on the formation of new blood vessels and influences the appearance of metastasis
[62]. Although it is a process controlled by a variety of factors, vascular endothelial growth factor A (VEGF-A) is among those mainly responsible
[40]. The human monoclonal antibody anti-VEGF-A Bevacizumab (Avastin, Roche Registration GmbH, Germany) is among the most prominent antiangiogenic drugs for angiogenesis inhibition
[63][64][63,64]. Despite causing many side effects, such as bleeding, skin rashes and hypertension
[65], Bevacizumab was approved in 2008 by FDA for the treatment of HER2-negative breast cancer in combination with paclitaxel (Taxol, Teva Pharma, Madrid, Spain) or capecitabine (Kern Pharma, Barcelona, Spain)
[66].
Cyclin-dependent kinases (CDKs), such as the kinase cyclin D/cdk4/6, are key enzymes in cell progression, tumor development and clonal expansion
[67]. CDK4/6 inhibitors, such as palbociclib (Ibrance, Pfizer, Ixelles, Belgium), ribociclib (Kisqali, Novartis Europharm Limited, Ireland) and abemaciclib (Verzenios, Eli Lilly Nederland B.V., Utrecht, The Netherlands), were approved in 2017 by the FDA for the treatment of HER2-positive or -negative breast tumors, in combination with endocrine therapy. Although this scheme can cause neutropenia as the main side effect, it is usually well tolerated and has led to a significant improvement in patient overall survival
[68].
The PI3K/Akt/mTOR pathway plays a fundamental role in cell proliferation, survival and development
[69], and is altered in breast cancer
[70]; therefore, efforts have focused on trying to inhibit the various components that make up this signaling pathway. The PI3K inhibitor alpelisib (Piqray, Novartis Europharm Limited, Ireland) was the first FDA-approved breast cancer drug for hormone-receptor-positive and HER2-negative patients. Its approval in 2020 was under its combined use with fulvestrant (AstraZeneca, UK), which is an estrogen receptor antagonist
[71], and its most common side effect is hyperglycemia
[72]. For its part, everolimus (Afinitor, Novartis Europharm Limited, Ireland), which is an inhibitor of the mTORC1 complex, was approved by the FDA in 2009 for patients with hormone-receptor-positive, HER2-negative advanced breast cancer in combination with exemestane (Exemestane Sandoz, Sandoz Farmacéutica, Madrid, Spain), which is a steroid aromatase inhibitor. Like Alpelisib, Everolimus causes hyperglycemia as a major side effect, as both affect lipid metabolism
[73].
Currently, for Akt kinase, the inhibitor ipatasertib (GDC-0068, RG7440) is still under development for the treatment of locally advanced/metastatic inoperable TNBC. In the preclinical phase, it demonstrated efficacy in inhibiting the PI3K/AKT pathway
[74]. In phase Ib, the combination of this drug with paclitaxel (Taxol, Teva Pharma, Spain) evidenced good tolerance
[75], while in phase II, it managed to improve tumor-progression-free survival
[76][77][76,77]. However, recent phase III results show that adding this drug does not improve the efficacy of treatment with paclitaxel (Abraxane, Bristol-Myers Squibb Pharma, Dublin, Ireland)
[78].
2. Role of the Ca2+-Signaling Pathway in Breast Cancer
Ninety-nine percent of the total body calcium is found in the body in mineral form as calcium hydroxyapatite (Ca
10[PO
4]
6[OH]
2) associated with hard tissues, such as bones and teeth, which also act as a reservoir and source of free calcium ions (Ca
2+) that are essential for bodily and cellular physiological functions
[85].
As a second messenger, intracellular Ca
2+ levels increase as a stimulus–response reaction, with an allosteric regulatory effect on enzymes and proteins involved in signal transduction pathways and different cellular processes, such as gene activation, secretion, migration, division, differentiation, proliferation and cell death
[86], as well as invasion, metastasis and acquisition of drug resistance
[87]. In the 1940s, a decrease in calcium levels in epidermal carcinoma cells was observed for several weeks, followed by the transformation of these cells into malignant ones, when this precancerous condition was experimentally induced
[88]. Since then, the central role of this ion and proteins involved in Ca
2+-signaling pathways in carcinogenesis and tumor progression has been widely reported
[89] in different types of malignancies, including breast cancer
[61][90][61,90], which is why blocking calcium signaling was proposed as a promising strategy to improve the efficacy of current anticancer therapies, as well as antitumor immune responses.
Calcium homeostasis is achieved by keeping cytosolic calcium levels low, with the extracellular space, cytoplasm, endoplasmic reticulum and mitochondria being the four primary compartments involved in cellular Ca
2+ circulation
[91], and with both the mitochondria and endoplasmic reticulum serving as intracellular calcium stores. Indeed, in the face of an extracellular Ca
2+ concentration of 1.3 mM
[92], cytoplasmic Ca
2+ in resting cells is maintained at concentrations ranging from 0.05 to 0.15 mM
[93][94][93,94], mainly due to the coordinated function of calcium receptors, organelle and membrane ion channels, membrane pumps and transporters, as well as calcium buffer proteins. The modulation of the Ca
2+ concentration is tightly regulated according to cellular needs
[92] by three main processes that are not mutually exclusive
[85]:
-
Amplitude modulation
[95]: the process responsible for triggering different downstream signaling responses, as proteins with higher Ca
2+ binding affinity are activated at lower Ca
2+ concentrations, whereas proteins with lower Ca
2+ binding affinity are activated at higher concentrations
[96].
-
Frequency modulation: the process by which repetitive and transient increases in cytosolic Ca
2+ concentration led to different protein activation
[95].
-
Modulation related to the spatial distribution of signals, which depends on the localization of effectors to Ca
2+ modulators, such as channels
[97].
Considering that the ion concentration in luminally mammary glands and breast milk is 10 mM and 2–4 mM, respectively, Ca
2+ homeostasis is especially important in mammary gland cells, even more so during the lactation process, with them being very sensitive to changes in Ca
2+ signaling, concentration and modulation mechanisms, which are also decisive in breast cancer progression
[98]. However, despite the importance of Ca
2+ during lactation and the association of dysregulation of calcium homeostasis and signaling with mammary gland pathophysiology, the implications of calcium signaling in the regulation of cell proliferation, differentiation and apoptosis are not yet fully understood
[99].