It is well known that blood lipoproteins (LPs) are multimolecular complexes of lipids and proteins that play a crucial role in lipid transport. High-density lipoproteins (HDL) are a class of blood plasma LPs that mediate reverse cholesterol transport (RCT)—cholesterol transport from the peripheral tissues to the liver. Due to this ability to promote cholesterol uptake from cell membranes, HDL possess antiatherogenic properties. This function was first observed at the end of the 1970s to the beginning of the 1980s, resulting in high interest in this class of LPs. It was shown that HDL are the prevalent class of LPs in several types of living organisms (from fishes to monkeys) with high resistance to atherosclerosis and cardiovascular disorders. Lately, understanding of the mechanisms of the antiatherogenic properties of HDL has significantly expanded. Besides the contribution to RCT, HDL have been shown to modulate inflammatory processes, blood clotting, and vasomotor responses. These particles also possess antioxidant properties and contribute to immune reactions and intercellular signaling.
- high-density lipoproteins
- HDL functions
- reverse cholesterol transport
- miRNA
- inflammation
- nanoparticle
1. Introduction
Lipoproteins were initially defined according to their composition (i.e., lipids and proteins) and classified according to their density (from very low- to high-density lipoproteins (HDL)). Historically, the most interest in HDL was linked to the negative correlation between HDL cholesterol (HDL-C) level and risk of coronary artery disease (CAD), as established by the Framingham study, concluding that low HDL-C levels are as much a risk factor for CAD as high low-density lipoprotein cholesterol (LDL-C) levels [1]. These data were later confirmed by other studies [2][3][2,3], and it is believed that they can be explained by the role of HDL in reverse cholesterol transport (RCT) from the macrophages in peripheral tissues to the liver for disposal [4].
Although it was originally believed that the level of HDL-C was the most important determinant of these processes, HDL-C-raising therapies all failed to improve cardiovascular outcomes. Clinical trials of cholesteryl ester transfer protein (CETP) inhibitors were very disappointing and ended with a conclusion of “insufficient cardiovascular benefit for routine use” [5]. Indeed, emerging evidence indicates that the composition and, consequently, the function of HDL is perhaps the most important factor, rather than their quantity and that the HDL-C concentration reflects only one side of the story. Dysfunctional HDL are unable to protect blood vessels, and, therefore, new strategies to measure and restore HDL functionality are needed [6].
Nowadays, it is widely accepted that HDL are a class of natural nanoparticles that display pleiotropic functions, such as RCT, anti-inflammatory action, immunity, antioxidative effects, and antithrombotic action [7][8][9][10][7–10]. Proteomics, lipidomics, and the analysis of small RNA in HDL particles are uncovering more and more of the functions of HDL. For instance, it has been established that HDL are complex particles that undergo dynamic remodeling through interactions with various enzymes and tissues throughout their life cycle, making the complete understanding of their functions and roles more complicated than initially expected [11].
The great interest in HDL pertains to the understanding of their functions from an evolutionary point of view, which shows that these ancient blood particles are not only lipid transporters but also display important functions in many aspects of immunity, tissue homeostasis, and intercellular signaling in both invertebrates and vertebrates. The protective effects of HDL in cardiovascular disease rely on this broad spectrum of homeostatic properties.
It seems that the time has come for us to leave our canonical views of HDL as lipid transporters and to crystallize the new data on HDL pleiotropic functions in favor of a new concept of HDL as an ancient dynamic platform for maintaining many aspects of an organism’s homeostasis. It is obvious that some of the protective properties of HDL can be used to improve the treatment of many acute and chronic diseases and to develop new HDL-based medicines.
In this review, we summarize the data on the dynamic structure and pleiotropic functions of HDL to update the information about them and highlight the potential for the therapeutic use of HDL’ functionality as a prospective biomimetic therapeutic platform.
2. Composition and Structure of HDL
“HDL” were discovered in 1929 when a lipid-rich α-globulin was isolated from horse serum by Macheboeuf at the Pasteur Institute in Paris [12]. HDL are a family of particles that can exhibit fundamentally different metabolisms and functions based on their specific proteomic, lipidomic, and physicochemical properties. HDL are the smallest lipoproteins (LPs) of blood serum with a density of 1.063–1.210 g/mL and a particle size of 7–12 nm, and, according to modern terminology, they are ultrasmall natural nanoparticles. The structure of HDL is similar to all LPs: HDL consist of the hydrophobic nucleus of non-polar lipids (triglycerides and esterified cholesterol) surrounded by a monolayer of phospholipids and free cholesterol, with inclusions of protein molecules named apoproteins. HDL are a heterogeneous fraction of LPs with several subfractions of various densities, sizes, forms, electrophoretic mobilities, protein–lipid compositions, and physiological functions [13][14][13,14]. The first method of separation of blood plasma LPs to fractions was electrophoresis in agarose gel. HDL are characterized by higher mobility compared to other LPs and thus produce a separate band marked as α-LPs. The band of α-LP fractions differs from other bands of larger and lighter LPs of other classes, such as β- and preβ-LPs.
The wide heterogeneity of HDL fractions—the difference in the ratio of lipid and protein components—and the resulting differences in the densities and sizes (diameter) of the particles determine the differences in the principle of their classification. According to Jomard and Osto [11], the most convenient for clinical usage is nuclear magnetic resonance (NMR)-based HDL classification [15], identifying five distinct HDL subfractions [16]: very large HDL particles, large HDL particles, medium HDL particles, small HDL particles, and very small HDL particles. This nomenclature also includes an entry for the pre-β-1 HDL subclass that participates in macrophage cholesterol efflux [15]. Despite the application of this approach in a number of works [17], in most studies, the same fractions were separated by ultracentrifugation, based on differences in their densities, and, similar to other plasma LPs, the density of HDL molecules serves as the main criterion of their classification. Ultracentrifugation is also the main method for the separation of α-LP subfractions. Based on the differences in particle densities, HDL2 and HDL3 fractions with densities of 1.063–1.125 and 1.125–1.21 g/mL, respectively, have been isolated. In a number of works, along with this, deeper fractionation of each of these two fractions has been carried out. In accordance with the results of electrophoretic separation of HDL subfractions in gradient non-denaturing polyacrylamide gel electrophoresis (GGE), they can be separated into subfractions with different molecule sizes. Two HDL2 and three HDL3 subclasses have been identified and their particle size characterized by this method: HDL3c, 7.2–7.8 nm diameter; HDL3b, 7.8–8.2 nm; HDL3a, 8.2–8.8 nm; HDL2a, 8.8.–9.7 nm; and HDL2b, 9.7–12.0 nm [18] [18] (Table 1).
Table 1. Main subclasses of high-density lipoproteins (HDL) (adapted from [18]).
|
Density, g/mL |
HDL Separation by Ultracentrifugation |
HDL Fractions Separation by Electrophoresis |
||
|
HDL Fractions |
Diameter, nm |
HDL Subfractions |
Diameter, nm |
|
|
1.063–1.125 |
HDL2 |
8.8–12 |
HDL2b * |
9.7–12 |
|
|
|
|
HDL2а * |
8.8–9.7 |
|
|
|
|
|
|
|
1.125–1.21 |
HDL3 |
7.2–8.8 |
HDL3а * |
8.2–8.8 |
|
|
|
|
HDL3b * |
7.8–8.2 |
|
|
|
|
HDL3с * |
7.2–7.8 |
|
|
|
|
|
|
|
> 1.21 |
preβ-HDL |
|
preβ-HDL ** |
|
* Gradient non-denaturing polyacrylamide gel electrophoresis (GGE); ** agarose gel, two-dimensional gel electrophoresis (2-DE).
The division of HDL into separate big (HDL2), medium (HDL3a), and small (HDL3b + HDL3c) subfractions by particle size has been confirmed by NMR-spectroscopy [19] [19] and matches the above-proposed classification of five subfractions based on size [11]. Moreover, the additional LP fraction, also considered as a class of HDL, has been revealed in plasma fractions with a density of more than 1.21 g/mL. These LPs are pre-beta-migrating when separated by agarose gel electrophoresis and are called preβ-HDL. Apoprotein A-I (ApoA-I), which is present in all HDL fractions, is the main component of this subfraction [20], which is lipid-poor and gradually enriched by lipids in circulation.
Preβ-HDL are synthesized in the liver and small intestine. They consist of ApoA-I, phospholipids, and free cholesterol released from cells. Due to the absence of non-polar lipids in their structure, preβ-HDL form disk-shaped structures, called newly developed or nascent HDL [21]. Nascent HDL are quickly transformed into spherical HDL during the circulation and esterification of cholesterol. Cholesterol esterification is catalyzed by lecithin–cholesterol acyltransferase (LCAT), and disk-shaped nascent HDL may be revealed in plasma only in familial LCAT deficiency. Cholesterol esters generated during this reaction form the hydrophobic nucleus of HDL particles. This process is accompanied by the production of small HDL3 subfractions following the transformation of these particles [22][23][22,23].
The high dynamics of HDL subfractions in blood serum, a constant exchange of lipid and protein compounds, and the transformation of fractions into one another during HDL remodeling should be emphasized. In fact, the boundaries of the densities and sizes of HDL subfractions given in Table 1 are somewhat arbitrary, and there are no clear boundaries between them, unlike other, lighter classes of LPs. In the process of the circulation of the smallest particles, starting from HDL3 and even earlier (preβ-HDL), they are gradually enriched with lighter (compared to protein) lipid components, which can also be exchanged with other blood components while increasing in size. The density of the particles is gradually reduced, and HDL turn into larger and lighter HDL2b. The ratio between different HDL subfractions indirectly reflects the intensity of these transformation processes, called remodeling [24]. The information about HDL remodeling remains incomplete, but it has been shown that this process is related to certain functions of these LPs. The analysis of HDL by modern proteomic mass spectrometry methods has allowed the identification of several short-living subfractions formed due to the temporary association of HDL particles with certain plasma proteins among the abovementioned fractions [25].
The content of protein molecules in HDL is approximately 50% of the particle weight. More than 90% of HDL proteins are composed of ApoA-I and ApoA-II proteins; the ratio of ApoA-I and ApoA-II in the HDL2 and HDL3 is approximately 3:1, while ApoC composes only 3%–5% of the proteins of HDL2 and 1%–2% of the proteins of HDL3. However, approximately half of ApoC observed in blood plasma under fasting conditions is a part of HDL. HDL are considered a depot of ApoC, which is released from chylomicrons and very-low-density lipoproteins (VLDL) during lipolysis [26]. Besides the abovementioned apolipoproteins, HDL contain other minor newly observed apoproteins, as well as an enzyme paraoxonase [27].
Several enzymes and transport proteins mediating lipid metabolism, which are bound to HDL, are present in plasma. The association of these molecules and HDL has been shown by mass spectrometry-based proteomics with high sensitivity. HDL fractions contain up to 80 of these proteins [27]. The main proteins permanently or temporarily bound to HDL, as well as their properties and functions, are shown in Table 2.
Table 2. Basic HDL proteins and their functions (adapted from [18]).
|
Protein |
Origin and Biological Function |
|
ApoА-I |
The main structural and functional apolipoprotein, which interacts with cellular receptors, activates lecithin–cholesterol acyltransferase (LCAT) and exhibits antiatherogenic activity. The main sites for ApoAI synthesis and secretion are the liver and small intestine. |
|
ApoА-II |
Structural and functional apolipoprotein, predominantly synthesized in the liver. |
|
ApoА-IV |
Structural and functional apolipoprotein, synthesized in the intestine. |
|
ApoС-I |
Possesses a high positive charge and, thus, can bind free fatty acids, can modulate the activity of some of the proteins involved in HDL metabolism, can activate LCAT, and can inhibit hepatic lipase and cholesterol ester transport protein (CETP). |
|
ApoС-II |
Activates lipoprotein lipase (LPL). |
|
ApoС-III |
LPL and hepatic lipase inhibitor. |
|
ApoС-IV |
Regulator of triglyceride (TG) metabolism. |
|
ApoD |
Responsible for the binding and transport of small hydrophobic molecules. Expressed in many tissues, including the liver and the intestines. |
|
ApoE |
Structural and functional apolipoprotein, a ligand for low-density lipoprotein (LDL) receptors and LDL receptor-associated protein (LRP), and binds to glycosaminoglycans on cells. Synthesized in several tissues and cell types, including the liver, endocrine tissues, central nervous system, and macrophages. |
|
ApoF |
Inhibitor of cholesterol ester transport protein (CETP). It is synthesized in the liver. |
|
ApoH |
Binds negatively-charged molecules, primarily cardiolipin, and prevents the activation of the blood coagulation cascade by binding to phospholipids on the surface of damaged cells. Regulates platelet aggregation and is expressed in the liver. |
|
ApoJ |
Binds hydrophobic molecules and interacts with cell receptors |
|
ApoL-I |
The main component of the serum trypanolytic factor. It is expressed in the pancreas, lungs, prostate, liver, placenta, and spleen. |
|
ApoM |
Binds small hydrophobic molecules, primarily sphingosine-1-phosphate (S1P), as well as oxidized phospholipids. It is synthesized in the liver and kidneys. |
|
PON1 (paraoxonase 1) |
Са2+- dependent lactonase with antioxidant properties, mainly synthesized in the liver, but also in the kidneys and colon. |
The multiple effects of apoproteins ApoF, -H, -J, -L, and -M, despite their low content in HDL, reflect their high biological activity and influence on HDL functions [28]. It might also suggest that, besides the effects of the apoproteins listed in Table 2, some of these agents or their combinations might contribute to the maintenance of the surface structure of HDL. These apoproteins might also determine the temporary association of various functional proteins circulating in plasma with HDL particles, which are mentioned in Table 3.
Table 3. Proteins associated with HDL and their functions (adapted from [18]).
|
Protein |
Biological Function |
|
Enzymes |
|
|
LCAT (lecithin–cholesterol acyltransferase) |
Esterifies cholesterol to cholesterol esters. LCAT is mainly expressed in the liver and, to a lesser extent, in the brain and testes. |
|
PAF-AH (platelet-activating factor acetyl hydrolase; lipoprotein-associated phospholipase A2 (LpPLA2)) |
Hydrolyzes short-chain oxidized phospholipids. Synthesized in the brain, white adipose tissue, and placenta. Macrophages are the most important source of the circulating enzyme. |
|
GSPx-3 (glutathione selenoperoxidase 3) |
A component of the system of protection against the oxidative damage of molecules. Catalyzes the redox reaction of peroxides (hydrogen peroxide to water or lipid peroxides to the corresponding alcohols) with glutathione. It is synthesized in the liver, kidneys, heart, lungs, mammary glands, and placenta. |
|
Lipid transport proteins |
|
|
PLTP (phospholipid transfer protein) |
Remodels HDL into large and small particles and binds and transports bacterial lipopolysaccharide. It is synthesized in the placenta, pancreas, lungs, kidneys, heart, liver, skeletal muscles, and brain. It is also a positive marker of the acute phase of inflammation. |
|
CETP (cholesterol ester transport protein) |
Provides heteroexchange of cholesteryl ester (CE) and TG and homoexchange of phospholipid (PL) between HDL and ApoB-containing lipoproteins. It is synthesized in the liver and adipose tissue. |
|
Acute-phase proteins |
|
|
SAA1 (serum amyloid A1) |
Major acute-phase reactant. Formed preferably in the liver. |
|
SAA4 (serum amyloid A4) |
Minor acute-phase reactant. Formed preferably in the liver. |
|
Alpha-2-HS glycoprotein |
Negative acute-phase reactant, which promotes endocytosis and opsonization. It is synthesized in the liver. |
|
Fibrinogen alpha chain |
Fibrin precursor, main component of blood clots and platelet aggregation. |
|
Complement system proteins |
|
|
С3 |
One of the main activators of the complement system through classical and alternative paths. |
|
Proteinase inhibitors |
|
|
α-1-antitrypsin |
Inhibits serine proteases, especially neutrophil elastase. |
|
Hrp (haptoglobin-related protein) |
Decoy substrate to prevent proteolysis. |
|
Other proteins |
|
|
Transthyretin |
Thyroid hormone binding and transport. |
|
Serotransferin |
Iron binding and transport. |
|
Vitamin D-binding protein |
Vitamin D binding and transport. |
|
α-1B-glycoprotein |
Unknown. |
|
Hemopexin |
Heme binding and transport. |
The high number of associated HDL proteins, along with the wide spectrum of functions, determine the multifunctionality of HDL and their contribution to various biological processes. For example, the level of the serum amyloid protein SAA1 in HDL negatively correlates with the ability to uptake cholesterol [29][30][31][29]. Modifications of HDL proteins, particularly the main protein ApoA-I, are observed under certain conditions. In particular, oxidized methionine in the 148 position of this protein has been shown in patients with cardiovascular disorders and type II diabetes. The observed impairments might be associated with the dysfunction of HDL [32][33][30,31]. Proteomic analysis of HDL in vitro exposed to acrolein (an aldehyde of cigarette smoke) has shown the appearance of cross-links between apoproteins and acrolein adducts of ApoA-I and ApoA-II. The authors proposed that these changes might contribute to the atherogenic effects of smoking by the reduction in HDL activity [34][32].
3. Functions of HDL
The further functions of HDL in organisms are discussed in this section in detail.
3.1. Reverse Cholesterol Transport (RCT)
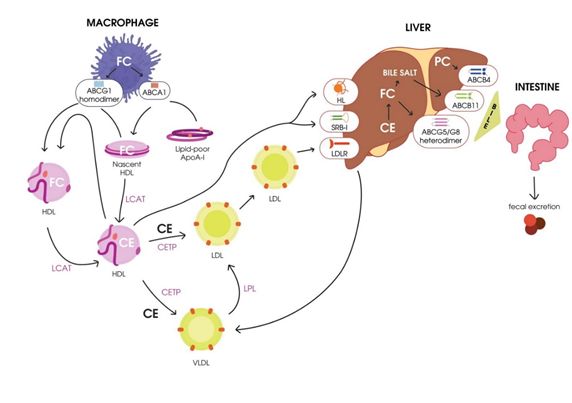
RCT, the transport of excess cholesterol from the peripheral tissues to the liver for utilization and excretion with the bile [35][33], is considered a classical function of HDL. The term RCT was first proposed in 1968 [36][34], and in accordance with Glomset’s hypothesis, excess free cholesterol is toxic for cells. Cells of peripheral tissues cannot excrete excess cholesterol, and its accumulation in the endoplasmic reticulum (EPR) might result in protein folding impairments, thus triggering apoptosis. To prevent these processes, excess cholesterol should be removed from the cells by RCT [20]. The main stages of RCT were originally described by Havel and Small in 1987 [37][38][35,36] and then later investigated [39][37–41], and are as follows:
- Cholesterol mobilization and transport to the plasmatic membrane of the cell.
- Transport of free cholesterol from the plasmatic membrane to acceptor particles (HDL) by the participation of membrane-localized ATP-binding cassette transporters ABCA1 and ABCG1 [37]. A significant role [39]. A significant role in this process belongs to the main phospholipid (PL) of HDL, phosphatidylcholine [30]in this process belongs to the main phospholipid (PL) of HDL, phosphatidylcholine [42].
- Esterification of free cholesterol bound to HDL by LCAT.
- Transport of cholesterol esters to hepatocytes by mature HDL.
- Engulfment of cholesterol esters from mature HDL particles mediated by the hepatocyte scavenger receptor class B type I (SR-BI) [43].[38].
- Transport of cholesterol esters to hepatocytes by mature low-density lipoproteins (LDLs) due to cholesterol exchange to HDL mediated by
A scheme of RCT is presented in Figure 1.
Figure 1. Schematic representation of reverse cholesterol transport. HDL, high-density lipoprotein; CETP, cholesterol ester transport protein; LCAT, lecithin–cholesterol acyltransferase; FC, free cholesterol; CE, cholesteryl ester; HL, hepatic lipase; VLDL, very-low-density lipoprotein; LPL, lipoprotein lipase; LDL, low-density lipoprotein; LDLR, LDL receptor; PC, phosphatidylcholine; SR-BI, scavenger receptor class B type I.
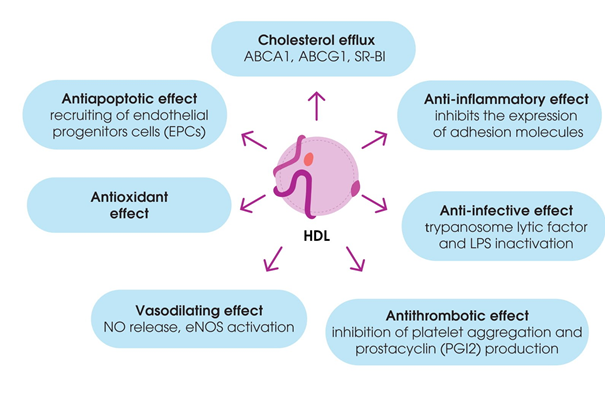
3.2. Non-Classical Functions of HDL
Besides their contribution to RCT, HDL have been shown to have antioxidant properties to modulate inflammatory responses, vasomotor reactions, and blood clotting, as well as to mediate immune responses. These functions of HDL are often impaired during dyslipidemia and atherosclerosis [31][43] (Figure 2).
Figure 2. Pleiotropic effects of high-density lipoproteins (HDL).