You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Emmanuel Nii Adjei Annan and Version 2 by Lindsay Dong.
Wheat (Triticum spp.) is a cereal crop domesticated >8000 years ago and the second-most-consumed food crop nowadays. Ever since mankind has written records, cereal rust diseases have been a painful awareness in antiquity documented in the Old Testament (about 750 B.C.). The pathogen causing the wheat stem rust disease is among the first identified plant pathogens in the 1700s, suggesting that wheat and rust pathogens have co-existed for thousands of years. With advanced molecular technologies, wheat and rust genomes have been sequenced, and interactions between the host and the rust pathogens have been extensively studied at molecular levels.
- wheat
- rust
- effector
- co-evolution
1. History of Wheat-Rust Co-Existence
Wheat refers to the cultivated Triticum spp., including the most common hexaploid bread wheat (T. aestivum L.) and the tetraploid durum wheat (T. turgidum var. durum). These cereal crops are the staple food in most developing countries and are the most traded grains globally. Wheat rust is among the earliest documented plant diseases, dating back to Aristotle’s time (384–322 B.C.) [1]. Epidemics of rust diseases were a reason for an ancient practice of Robigus, the rust god [2], and now the diseases are still one of the major constraints for wheat production worldwide [3]. There are three fungal species from the genus of Puccinia, P. triticina (Pt), P. graminis f. sp. tritici (Pgt), and P. striiformis f. sp. tritici (Pst) causing leaf, stem and stripe rust on wheat, respectively. The Pgt was first described with details by two Italian scientists, Fontana and Tozzetti, independently in 1767 and named by Persoon in 1797 [1]. Anton De Bary found barberry as an alternative host of Pgt in 1865 [1]. The three species of rusts are obligate biotrophic parasites that require living host cells to grow and reproduce [4]. However, the parasites can remain alive as spores in the absence of a living host for a period of time depending on the conditions [5]. Five types of spores are produced by the three rust pathogens, production of urediniospores, and teliospores on the primary grass hosts such as wheat by asexual reproduction. Teliospores germinate to produce basidiospores infect an alternative host to form pycniospores (spermatia) and produce aeciospores by sexual reproduction on the alternative host [6][7][6,7].
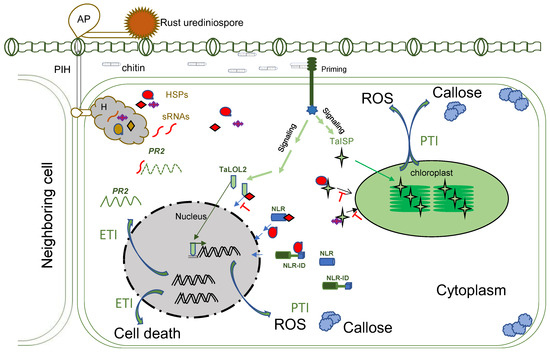
2. No Interactions during Rust Germination
Rust infections on their primary grass hosts, including wheat, start from aeciospores/urediniospores landing on the leaf surfaces. Under moist conditions, free water is required for spore germination, a process that occurs at night in the natural environment. Expression analysis using a cDNA library from germinated Pst urediniospores revealed germination stage-specific-expressed genes [8][9]. Over 60% of the genes were involved in primary metabolism (42.6%) and protein synthesis (21.6%). Some of the stage-specific-expressed Pst genes shared significant homology with known virulence factors such as HESP767 of flax rust and PMK1, GAS1, and GAS2 of rice blast fungus [8][9]. The germination of spores does not require living hosts, and the process can happen on a plastic surface when free water is present [8][9]. However, germination is inhibited by endogenous self-inhibitors released from the spores if the population density floated on the water is too high [9][10]. Each rust species has its optimum germination temperature in the range of 11~20 °C. Neither host genotypes (resistant or susceptible) nor host extracts [10][11] affect spore germination rates, thus suggesting that host defense responses did not happen at the stage of spore germination before penetration [11][12].3. Molecular Interactions during Penetration and Haustoria Formation
After germination, the germ tubes grow perpendicular to leaf veins until they encounter stomata. The topology of specific host guard cells plays a vital role in stomata identification, known as thigmotropism. Formation of an appressorium is induced over a stoma around 4~16 h post-germination [12][13]. Then, an appressorium forms a penetration peg to initiate penetration, and a substomatal tube grows between the two guard cells of the host, a mechanism previously determined to be light-dependent [13][14]. Penetration is shortly followed by substomatal growth, including the formation of primary infection hyphae (PIH) that grow intracellularly until they encounter mesophyll cells. Once a PIH contacts a living mesophyll cell, the tip of the PIH differentiates to a haustorial mother cell (HMC), and a haustorium is induced (Figure 1). The HMC located outside of the mesophyll cell degrades a tiny hole on the cell wall and invaginate the plasma membrane to form an intracellular young haustorium [14][15] at about 24 h post-infection (hpi), and a mature haustorium as early as 48 hpi [15][16]. If the host cell in contact with the PIH is dying, the PIH differentiation stops, and hyphae will continue to grow to find another viable cell [15][16]. The mechanism to govern this process is still unknown.
Figure 1. Molecular interactions with wheat host during rust infection. AP: appressorium; PIH: primary infection hyphae; H: haustorium; HSPs: haustorial secreted proteins; sRNA: small RNA; PR2: pathogenesis-related protein 2; ROS: Reactive Oxygen Species; TaISP: Triticum aestivum iron-sulfur protein; TaLOLs: Triticum aestivum LSD-One-Like 2; NLR: nucleotide-binding and leucine-rich repeat; NLR-ID: NLR–integrated domain; PTI: pathogenic-associated molecular pattern-triggered immunity; ETI: effector-triggered immunity.
During penetration of the host cell, a fungal cell wall component chitin can be an elicitor to trigger the wheat defense response [16][17], with the signatures of bursts of reactive oxygen species (ROS) such as H2O2 and increased callose deposition around the penetration sites [17][18][18,19], known as pathogenic-associated molecular pattern-triggered immunity (PTI). The host PTI is regulated by priming, signal cascades, and movements of transcription factors to the nuclear to activate more genes or movements of nucleus-encoded proteins to chloroplasts to generate ROS and callose. Wheat transcription factors TaLOL2, TaCBF1d, and a copper–zinc superoxide dismutase TaCZSOD2 are known to be positively involved in PTI [19][20][21][20,21,22].
At the early penetration stage, rusts deploy effectors to interfere with different steps of host PTI operated by the nucleus or organelles, such as chloroplasts. For example, the Pst effector PstGSRE1, a glycine–serine-rich protein, disrupted the movement of TaLOL2 to the nucleus [20][21]. TaLOL2 is a transcription factor promoting ROS. PstGSRE4 targeted the wheat copper–zinc superoxide dismutase TaCZSOD2, an enzyme positively involved in PTI [19][20]. The PstPEC6 effector interacted with the wheat adenosine kinase to hamper ROS accumulation and callose deposition [22][31]. Genes encoding PEC6 homologs are conserved among Pst isolates and highly similar among three rust species [23][28], suggesting a conserved strategy for suppressing PTI among the rusts. Pst effector PsSpg1 interacted with TaPslPK1 to promote its nuclear localization and subsequent phosphorylation of TaCBF1d and degradation. TaCBF1d is a crucial transcription factor in activating PTI [21][22]. Inactivation of TaPslPK1 rendered wheat with broad-spectrum resistance to rusts [21][22].
Once the host PTI was suppressed, rust could form haustoria, and haustorial-specific/enriched genes could start to express or upregulate [24][37] as an effector repertoire. Transcriptomic studies of multiple isolates of each Pst, Pgt, and Pt revealed that the Pst had 1989 differential expressed genes in haustoria; 400 possess a secretion peptide, and >40% of the genes are involved in metabolic processes and translation [25][38], all six Pt races had 456 haustorial secreted proteins (HSPs) [26][39], and four different Pgt isolates had 520 HSPs [27][25].
In addition to the deployment of effectors to attack crucial components of PTI/ effector-triggered immunity (ETI), rust produces small RNAs (sRNA) as important pathogenicity factors to impair host immunity [28][40]. For example, A 21-nt microRNA of Pst (Pst-milR1) was found to bind the wheat PR2 gene to reduce the transcript abundance of the gene and suppress the host defense during infection. PR2 is a β-1, 3-glucanase with antifungal property [29][41]. High production of PR2 is the result of active ETI. Silencing the precursor of the Pst-milR1 resulted in wheat resistance to the pathogen [28][40]. A transcriptomic study on wheat–Pst interaction at 7 dpi discovered differential expression profiles of sRNAs from wheat and Pst [30][42]. More 35-nt and less 24-nt sRNAs from the Pst infection wheat than the uninfected plant. Pst produced abundant sRNAs almost entirely of 19–23-nt in sizes [30][42].
Accordingly, wheat has a sophisticated surveillance system to detect rust effectors and activates even stronger defense responses, known as ETI. For example, wheat Sr35 detects Pgt AvrSr35 and mediates resistance to stop the PIH growth before the formation of haustoria [31][43]. So far, there are 231 designated rust resistance genes [32][33][34][35][36][37][44,45,46,47,48,49], including 83 leaf rust resistance (Lr) genes, 64 stem rust resistance (Sr) genes and 84 stripe rust resistance (Yr) genes. So many rust resistance genes implicate a high variation from their counterpart.
The first cloned wheat rust resistance gene Lr21 [38][50] shed light on the defense mechanism of wheat ETI against leaf rust. Lr21 encodes a protein with nucleotide-binding and leucine-rich repeat (NLR) domains, mediating resistance with hypersensitive cell death and high pathogenesis-related protein (PR) productions. Up to now, 29 rust resistance genes have been cloned [39][40][41][42][51,52,53,54]. Twenty-three of the 29 R proteins (79%) belong to the NLR class. After the bread wheat whole genome was sequenced, sequence annotation revealed that the wheat genome contains ~2151 NLR-like genes [43][55]. These genes are arranged in 547 gene clusters and located at the distal ends of the chromosomes, known as recombination hotspots [43][44][55,56].
The NLR-like proteins identified in the wheat genome are classified as the majority to be the classical NLRs containing an N-terminal coiled-coil (CC)/TIR + NB + LRR domains [42][54] and some NLR fusion proteins, e.g., an NLR fused with integrated domain (ID) homologous with proteins of different functions [42][45][46][54,57,58]. The abundance and locations of the wheat NLR proteins and their structures left footprints on how the new genes were evolved, and what the possible modes of their defense actions are.
Classical NLRs are known to recognize pathogen effectors, also called avirulence (Avr) proteins, directly or indirectly [47][59]. Studies on the molecular interactions of three pairs of wheat Sr proteins and the matching Avr proteins, Sr35/AvrSr35 [31][43], Sr50/AvrSr50 [48][60], and Sr27/AvrSr27 [40][52], demonstrated that mutations at the DNA or expression level of the matching effector genes were the mechanisms of generating new Pgt virulence isolates. For example, the Sr35-mediated resistance stops the development of PIH before the formation of haustoria [31][43]. A transposon-mediated insertion in AvrSr35 created Sr35-virulent Pgt isolates with increased expression in a susceptible wheat line [31][43].
There are 28 different types of IDs that have been identified to be present in either the N- or C-terminus of an NLR in wheat [43][55]. These IDs include kinase and DNA-binding domains, which function as signal transduction. An ID of a kinase or a DNA-binding in an NLR may help the NLR to initiate defense signaling through phosphorylation of transcription factors by the biochemical activity of kinase [49][82] or move directly to the nucleus with DNA-binding to the promoters of genes involved in defense responses. However, an ID such as NPR1 fused with an NLR protein is likely to serve as a molecular bait or decoy. NPR1 is a key transcriptional regulator in plant defense [50][51][52][53][54][83,84,85,86,87]. Wheat has 6 members of NPR1-like genes located in homoeologous groups 3 and 7. The group 3 NPR1 genes regulate the salicylic acid (SA)-signaling pathway and the crosstalk between SA- and Jasmonic acid (JA)- pathways. The Ta7ANPR1 locus in wheat encodes two types of NLRs, NB-ARC, and NB-ARC + NPR1, through alternative splicing [46][58]. Together with a CC + NB-ARC gene at a head-to-head orientation, it monitors the integrity of NPR1 proteins. Mutations in the Ta7ANPR1 activated resistance to rusts [46][58].
