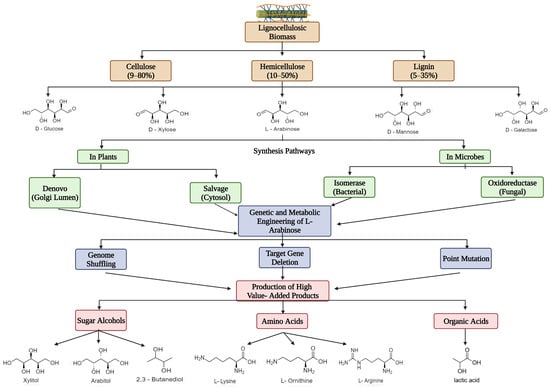
The exploration of natural substrates for microbial conversion to synthesize industrial platform and fuel chemicals seems to be inevitable within a circular bioeconomy context. Hemicellulose is a natural carbohydrate polymer consisting of a variety of pentose (C5) sugar monomers such as arabinose, mannose, erythrose, and xylose. Among the C5 sugars, L-arabinose (L-Ara) is the second-most-abundant pentose sugar in the lignocellulosic biomass after xylose. L-Ara has been used as an industrial carbon source to produce several value-added chemicals such as putrescine, which is used to synthesize polymers in the textile industry; sugar alcohols that are used as sweeteners in diet foods; and amino acids such as L-lysine, L-glutamate, L-arginine, and L-ornithine, which are used in nutritional supplements, fertilizers, and other products in the food and beverage industries. L-Ara, a natural non-caloric sweetener, is used as a substitute in the food and beverage industry, when the risk of blood sugar and lipid levels could be reduced. Major use of L-Ara is also found in the medical and pharmaceutical sectors to treat several conditions, including mineral absorption disorder, constipation, and diabetes, among others.
1. Introduction
In recent times, the term ‘Circular Bioeconomy’ is one of the keystones of the new economical and societal era to reverse climate changes and produce sustainable green chemicals from renewable carbon sources
[1]. Among the various renewable resource options, lignocellulosic biomass (LCB) seems to be the major contributor, with an annual production of 0.2 trillion metric tons
[2]. LCB also circumvents the food vs. fuel debate that is prominent among developing countries that reserve the most-abundant non-edible carbon feedstock either as an agro-industrial residue or as dedicated bioenergy crops. The plant biomass is mainly comprised of 65–85% of holocellulosic compounds (cellulose and hemicellulose) and 15–20% of lignin
[3][4][5][3,4,5]. Over recent decades, substantial efforts have been taken for the conversion of cellulosic-derived glucose into biofuels and other value-added products. Hemicellulose is a natural carbohydrate polymer consisting of a variety of pentose (C5) sugar monomers such as arabinose, mannose, erythrose, and xylose. Among the C5 sugars, L-arabinose (L-Ara) is the second-most-abundant C5 sugar in LCB after xylose. L-Ara is used as an industrial carbon source to produce several value-added chemicals such as putrescine (a polymer used in the textile industry), ethanol/sugar alcohols (artificial sweeteners in diet foods, fuel additives, etc.), amino acids such as L-lysine, L-glutamate, L-arginine, and L-ornithine (nutritional supplements), fertilizers, and other products in the food and beverage industries
[6][7][8][9][6,7,8,9]. Recent investigations revealed that the whole LCB could be an efficient resource for chemical and fuel production through a biorefinery framework, rather than only cellulose-based bio-renewables
[10]. Therefore, a sustainable utilization of LCB prerequisites a completely integrated biorefinery framework that is analogous to a petroleum refinery. In a biorefinery, the holocellulosic fraction contributes a prime role in the production of bio-renewables, owing to its efficient hydrolysis into monomeric sugars that could be subsequently fermented into an array of high-value-added commodities.
Table 1 represents a brief list of the value-added bioproducts that are produced. The global market value of food-grade L-Ara is expected to reach USD 33 million by 2028
[11]. In this regard, the valorization of L-Ara could be a promising alternative carbon source for industries that both economically and sustainably augment. There exists a bottleneck in the effective utilization of C5 sugars through microbial fermentation, wherein only a few industrially potent microbes are available for C5 sugar uptake through the specialized intramembrane transport mechanism and metabolic pathways, with a low product yield. However, the conventional metabolic pathway harbored by the microbial candidates possesses low-titer product yields. Hence, upgraded and adapted recent microbial technologies such as adaptive laboratory evolution (ALE)
[12][13][12,13], metabolic engineering, and synthetic biology
[14] have been recently emerging as a promising mitigation strategy to meet the industrial utilization of L-Ara for chemical synthesis and the purpose of establishing a sustainable greener technology
[1]. To the best of the authors’ knowledge, this is the first report to shed light on the significance of hemicellulose-derived L-Ara as a renewable carbon source and its valorization toward several value-added commodity chemicals. It also highlights the different metabolic pathways involved in the assimilation of L-Ara by various microbial candidates for industrially important chemicals. In addition, different research directions in terms of metabolic engineering, synthetic biology, and microbial strain improvement strategies are discussed.
Table 1.
Value-added products and their corresponding yields.
2. Abundance and Significance of L-Ara as a Bioresource
Hemicellulose, a heterogenous polymer that contains C5 sugars such as α- L-Ara and β-D-xylose, could reach 20–30% of the total LCB
[17][18][17,18].
Figure 1 represents the potential of LCB, its sugar composition, and its valuable application in industries through microbial metabolic processes. In addition, some other sugars such as α -fucose and α-L-rhamnose are also present to a small extent, albeit rarely
[19]. Based on the composition, presence, and side-chain ratio of the constituents, hemicellulose is distinguished as xyloglucan, glucuronoxylan, glucuronoarabinoxylan, galactoglucomanan, arabinoxylan, glucomannan, homoxylan, galactomannan, homomannan, arabinoxyloglucan, and arabinoglucuronoxylan. Among these, a considerable amount of L-Ara was found in arabinoglucuronoxylan, arabinoxyloglucan, glucuronoarabinoxylan, and arabinoxylan
[20]. Rapid growth in the fresh juice industry has led to the abundance of fruit processing waste, which is not being efficiently utilized. Fruit processing waste such as pear peel, lime peel, orange peel, mandarin peel, and apple pomace is rich in pectin, i.e., 12–35% of the biomass dry weight has an insignificant amount of lignin (2%,
w/
w), compared to that of LCB
[21][22][23][24][25][21,22,23,24,25]. Pectin is a complex heteropolysaccharide composed of α-1,4 linked D-galacturonic acid that contributes 70% of the total homogalacturonan polymer weight. When considering pectin, the presence of a limited amount of lignin merely enables the breakdown of polymers into monomers, where L-Ara becomes the most abundant of the C5 sugars. In addition to LCB, agro-industrial by-products such as wheat bran, corn fiber, sugar beet pulp, brewer’s spent grain, and sugarcane bagasse contain around 10.6%, 12.0%, 18.0%, 8.7%, and 1.3% of L-Ara, respectively
[26][27][28][29][30][31][26,27,28,29,30,31].
Table 2 represents the different feedstocks/sources of L-Ara and its potential industrial applications. These abundant waste resources could be sustainably tapped for the L-Ara waste production of chemicals through various microbial candidates, which is discussed in the subsequent sections.
Figure 1.
Production of value-added products from LCB-derived L-Ara.
Table 2.
Different sources of L-Ara and its applications.
2.1. 2,3-Butanediol
2,3-Butanediol (2,3-BD) or 2,3-butylene glycol has various applications, such as as a chemical feedstock, a solvent, a liquid fuel, and a raw material for several resins and synthetic polymers
[57]. A microorganism, identified as
Enterobacter cloacae, was found to produce meso-2,3-BD as its primary product during fermentation. There are reports that pathogenic bacteria and other microbes produced 2, 3-BD.
Klebsiella pneumoniae had the most significant 2, 3-BD titer of any bacterium, measuring 150 g/L
[58]. Another productive maker of 2,3-BD, classified as a class 2 bacteria, was
K. oxytoca, and this strain produced 2, 3-BD concentrations up to 130 g/L. Three bacteria with the Generally Recognized as Safe (GRAS) designation are effective 2, 3-BD producers:
Bacillus amyloliquefaciens,
B. licheniformis, and
B. subtilis. The discovery of new strains and the enhancement of optical clarity has received abundant interest. Industrially applicable hosts, such as
L. lactis,
Saccharomyces cerevisiae, and
Escherichia coli, are better-suited for large-scale production than indigenous hosts due to their effective genetics and well-proven cultivation techniques
[59].
In a study conducted by Saha and Bothast, the authors checked the production of 2,3-BD by
E. cloacae NRRL B-23289 by utilizing each of the following carbon sources individually: xylose, glucose, and L-Ara. The study was conducted at a pH of 5.0, a temperature of 30 °C, and 200 rpm. It showed that
E. cloacae NRRL B-23289 utilizes the above-mentioned carbon sources in the order: xylose < glucose < L-Ara. About 0.37 g of glucose and 0.38 g of xylose were consumed in 63 h, and 0.43 g of L-Ara was consumed in 39 h. The bacteria were cultivated on mixtures of A and B, made of sugar, in proportions of 1:1:1 and 1:2:1 for glucose, xylose, and L-Ara, respectively. The bacterium variety was found to favor glucose over xylose and L-Ara over xylose. After a significant amount of L-Ara was consumed and only after all of the glucose was used up, the xylose started to vanish. Thus, the authors could use the
E. cloacae NRRL B-23289 strain for the enhanced production of 2,3-BD using L-Ara as a carbon source
[60].
For an array of sectors, including those in the chemical, cosmetics, agriculture, and medicine fields, 2,3-BD holds enormous potential. 2,3-BD has broad industrial applications, such as as a promising bulk chemical, which has plenty of further use. Its high heating value makes it an excellent drop-in fuel. It can also be converted to octane after adding the methyl ethyl ketone (MEK) and hydrogenation reaction, which is then used to produce superior aviation fuel. It is widely used to manufacture antifreeze agents, pharmaceuticals, synthetic rubber, fumigants, foodstuffs, perfumes, fuel additives, and printing inks
[61].
2.2. Other Value-Added Products
For the past two decades, the market value for amino acids such as L-tryptophan, DL-methionine, L-lysine, L-aspartic acid, L-threonine, and L-glutamic acid has drastically increased owing to their wide range of applications in the food, cosmetics, agriculture, and pharmaceuticals sectors
[62]. Recent studies reported the utilization of hemicellulose-derived L-Ara as the sole carbon source by engineering microbial strains for organic acids (lactic acid and succinic acid) and amino acids production
[7][63][64][7,63,64]. Metabolic engineering of the
Corynebacterium glutamicum ATCC 31831 strain resulted in the production of L-amino acids, namely, L-ornithine, L-lysine, L-threonine, L-methionine, L-glutamate, diamine putrescine (1,4-diaminobutane), and organic acids upon arabinose transporter gene (araE) expression
[6][7][65][6,7,65]. On the other hand, overexpression of the ornithine decarboxylase gene (speC) from
E. coli resulted in a high yield of putrescine by the
C. glutamicum strain
[16].