The food industry produces an exorbitant amount of solid waste of petrochemical origin as a result of the increase in the development of new products. Natural polymers are an alternative to this theme; however, their development with adequate properties is a challenge. The union of different polymers in the synthesis of packaging is usually carried out to improve these properties. The combination of agar-agar and chitosan biopolymers show particular advantages through hydrogen bonds and electrostatic attraction between oppositely charged groups, presenting a promising source of studies for the synthesis of green packaging. When combined with natural extracts with active properties, these polymers allow an increase in the microbiological stability of foods associated with lower chemical preservative content and greater environmental sustainability.
- blend
- properties
- polymer matrix
- natural polymer
1. Introduction
2. Food Packaging
Food packaging is intended to allow transport, distribution, and handling, ensuring protection against shocks and compression. It works by minimizing product losses due to deterioration through the control of humidity, oxygen, light, and microbial development by acting as a barrier to the surrounding atmosphere [1]. Packaging must have performance compatible with its functionality, meeting the four basic functions of protection, communication, convenience, and containment while taking into account the characteristics of each product. It requires good mechanical strength, flexibility, and elasticity in order to avoid tears and perforations during all stages of production, storage, and marketing of the product [2]. Packaging is considered a vehicle for selling and promoting the brand, as it is the consumer’s first contact with the product, becoming one of the main characteristics for the decision at the time of purchase [17][16]. The packaging must be composed of inert material to ensure that there is no migration of its compounds to the food and that it does not pose a risk to the consumer’s health and/or change its sensory characteristics [15]. The vast majority of food packaging originates from polymers of petrochemical origin, which are popular due to their flexibility and lightness; however, petrochemical products represent a non-renewable resource. Thus, their use results in socioeconomic problems such as increased oil prices and the generation and accumulation of waste that can take tens or hundreds of years to decompose in nature [7]. The production of solid waste from food packaging has grown at a rate of 4.2% per year since 2010. It is estimated that this will continue until at least 2024 [18][17]. Considering all of the materials used in the development of these packaging, plastic corresponds to the largest share. Single-use packaging accounts for an important share of the millions of tons of plastics that end up in the oceans annually [19][18]. In 2018, 1.130 billion items of food and beverage packaging were sent to landfills in the European Union alone [20][19]. The consumption of plastic film has increased greatly in recent years; in combination with their long period of decomposition, phthalic acid esters (PAEs) are used as a plasticizer in the production of polyvinyl chloride (PVC) plastic films, and have a carcinogenic effect to living beings [21][20]. PVC plastic films are widely used on a daily basis, and in view of this problem, alternative means have been sought to reduce such impacts through the development of bioplastics and through the search for new technologies [8].3. Biopolymers
3.1. Agar-Agar
Agar-agar is a biopolymer belonging to the natural polysaccharides extracted from red algae of the Rhodophyta class, being the structural carbohydrate of the wall of these cells. It is composed of agarose, which has a straight chain, and agaropectin, which has a branched chain, linked together by bonds α- (1 → 3) e β- (1 → 4) [29][21]. It is an attractive biopolymer due to its chemical structure, resistance to acids, and ability to form a consistent gel even at low concentrations, favoring its application in several industrial areas [30][22]. In view of its various applications, the food sector stands out, where it is used as a thickener and in food packaging [31][23]. In agriculture, it acts as a soil conditioner and water absorber, and is very efficient in places with little water availability [32][24], while in medicine it is used in the microencapsulation of medicines and bioactive compounds [33][25]. In addition to having a highly porous matrix, it is interesting for particle trapping [29][21]. Films based on this polymer, however, are brittle in nature, have poor mechanical properties, and are highly sensitive to water, as they are hydrophilic in nature, which limits their application with high-moisture products [10]. However, there are studies that have used blending or reinforcement of this polymer for a final result with different characteristics, whether chemical, physical, or mechanical. Jridi et al. [34][26] used a combination of gelatin and agar, resulting in mechanically stronger films. Wongphan and Harnkarnsujarit [35][27] obtained improvements in the solubility of the film when developed using mixtures of starch, agar-agar, and maltodextrin.3.2. Chitosan
Chitosan is derived from chitin, and was discovered in 1859 when Rouget cooked chitin (itself discovered in 1811) in potassium hydroxide and found that it became soluble in organic acids [36][28]. Chitin is the second most abundant natural polymer in nature, and is obtained mainly from crab and shrimp shell residues, generally used in seafood industries. However it can be obtained from several other sources as well, such as mollusc shells, fungal cell walls and membranes, cell walls of algae, and the exoskeletons of insects and arachnids [36][28]. The deacetylation of chitin occurs by the transformation of acetamide (NHCO3) into amine (NH2) in a basic medium, being produced under different degrees of deacetylation and molecular weights that vary based on the alkaline concentration, time, and temperature used in the process [37][29]. Chitosan is the only polysaccharide of alkaline nature, the others being of acidic or neutral origin. It is a non-toxic, biocompatible, and biodegradable compound, and is absorbed by the body [26][30]. Chitosan’s properties are directly linked to its molecular weight, degree of deacetylation, and degree of crystallinity. Properties such as viscosity, solubility, tensile strength, and elongation are influenced by molecular weight, which corresponds to the number of sugar units per polymer molecule; the viscosity of chitosan solution is increased with increasing degree of deacetylation [37][29]. Chitosan polymers are aminopolysaccharides with unique structures, having several properties and high functionality, and can be applied to many diverse areas, both industrial and biomedical [38][31]. It is one of the most promising polymers of biological origin, and can be used as a food additive in the diet [39][32], in medicines, where it has great potential as an antacid and for protecting the stomach from other drugs in addition to acting as a transporter and drug releaser in the human body [40][33], and in cosmetics for the treatment of hair and skin, where it acts as a hydrating agent and has the ability to adhere to fragrance [41][34]. It has reported antiviral properties [42][35], in addition to acting as an antimicrobial agent. In this context, it acts on the external surface of bacteria, such as the cell walls of Gram-negative microorganisms (composed of lipopolysaccharides), on the peptidoglycan associated with teichoic acid, and on the cell membranes of Gram-positive bacteria [43][36]. However, chitosan has disadvantages for applications in bioplastic films when used as the sole source of polymer, as it has low solubility, which does not allow for interaction with other compounds often used to make films, such as plasticizers [21,40][20][33]. The union of chitosan with other polymers, however, results in a film with excellent characteristics [36][28]. Ghaderi et al. [44][37] obtained improvements in the barrier properties and solubility of films based on chitosan and vinyl alcohol when fish gelatin was added. Mendes et al. [45][38] produced films with better extensibility and thermal stability using a mixture of chitosan and corn starch. Li et al. [21][20] developed chitosan and sodium alginate films with good mechanical and hydrophobic properties as well as high light blocking capacity.4. Agar and Chitosan Blends
Agar is composed of a mixture of agarose and agaropectin, which correspond to the gelling and non-gelling fractions, respectively. In the agar-agar industrialization process, a large part of the agaropectin is removed, making a powder with greater gel strength [10]. The film formation process begins by (i) the formation of a viscous fluid through the dissolution of agar-agar powder, water, and gelation temperature (90–103 °C); (ii) cooling; (iii) thermoreversible gel formation; and (iv) evaporation. Gelation chemically occurs by the formation of hydrogen bonds between agarose molecules, forming a network of agarose double helices stabilized by water molecules [62][39]. In the drying process, the films have a high rate of retraction caused by the syneresis of the gel. The Agar-agar films allow easy interaction with mainly aqueous bioactive extracts. In film synthesis, when the solvent is replaced by an aqueous extract, it allows a homogeneous interaction with the agar-agar matrix [63][40]. Due to the high compaction of pure agar film, it becomes very brittle. A promising alternative to overcome this limitation is a combination with other biopolymeric substances. Chitosan films are synthesized from the dissolution of chitosan in aqueous solutions of organic acids, and have gained much attention from researchers due to their biocompatibility. Chitosan is used in combination with natural polymers such as starch and gelatin in order to improve its properties. It easily allows hydrogen bonds with these polymers, which makes it biocompatible [31][23]. Films developed with pure biopolymers have insufficient mechanical properties for application as packaging compared to films that use a mixture of polymers. The electrostatic interactions between agar-agar and chitosan are compatible, allowing for the production of stable films with good properties. The electrostatic interaction is caused by the –NH3+ groups of chitosan and –COO− of the ester group of agar-agar [64][41] in addition to intermolecular interactions through hydrogen bonds between functional groups, such as –OH [65][42] (Figure 21). The bond between the polymers allows synthesis of a homogeneous film that combines the benefits of both, depending on the concentrations used and the presence or absence of extracts, resulting in materials with particular properties [63][40].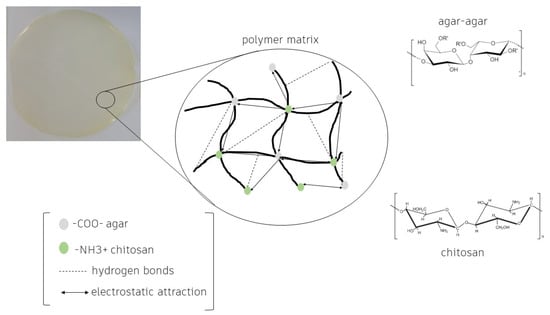
References
- Jadhav, E.B.; Sankhla, M.S.; Bhat, R.A.; Bhagat, D.S. Microplastics from food packaging: An overview of human consumption, health threats, and alternative solutions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100608.
- Rydz, J.; Musiol, M.; Zawidlak-Wegrzynsk, B.; Sikorska, W. Chapter 14—Present and Future of Biodegradable Polymers for Food Packaging Applications. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 431–467.
- Jiménez-Rosado, M.; Zarate-Ramírez, L.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Bioplastics based on wheat gluten processed by extrusion. J. Clean. Prod. 2019, 239, 117994.
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic contamination of the food chain: A threat to human health? Maturitas 2018, 115, 64–68.
- Golwala, H.; Zhang, X.; Iskander, S.M.; Smit, A.L. Solid waste: An overlooked source of microplastics to the environment. Sci. Total Environ. 2021, 769, 144581.
- Onu, Organização das Nações Unidas. 2019. Available online: https://nacoesunidas.org/onu-meio-ambiente-aponta-lacunas-na-reciclagem-global-de-plastico/ (accessed on 30 March 2023).
- Provin, A.P.; Dutra, A.R.A.; Gouveia, I.C.A.S.S.; Cubas, A.L.V. Circular economy for fashion industry: Use of waste from the food industry for the production of biotextiles. Technol. Forecast. Soc. Chang. 2021, 169, 120858.
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546.
- Moeini, A.; Germamm, N.; Maliconico, M.; Santagata, G. Formulation of secondary compounds as additives of biopolymer-based food packaging: A review. Trends Food Sci. Technol. 2021, 114, 342–354.
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176.
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; D’Ayla, G.G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839.
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.-H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536.
- Cheikh, D.; Majdoub, H.; Darder, M. An overview of clay-polymer nanocomposites containing bioactive compounds for food packaging applications. Appl. Clay Sci. 2022, 216, 106335.
- Latos-Brozio, M.; Mazek, A. The application of natural food colorants as indicator substances in intelligent biodegradable packaging materials. Food Chem. Toxicol. 2020, 135, 110975.
- Zhao, X.; Ji, K.; Kurt, K.; Cornish, K.; Vodovotz, Y. Optimal mechanical properties of biodegradable natural rubber-toughened PHBV bioplastics intended for food packaging applications. Food Packag. Shelf Life 2019, 21, 100348.
- Schifferstein, H.N.J.; Lemke, M.; Boer, A. An exploratory study using graphic design to communicate consumer benefits on food packaging. Food Qual. Prefer. 2022, 97, 104458.
- All4pack. Packaging: Market and Challenges in 2016. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjT3sLFkdXiAhWO4IUKHcYsCb4QFjAAegQIARAC&url=https%3A%2F%2Fwww.all4pack.com%2FMedia%2FAll-4-Pack-Medias%2FFiles%2FFiches-marches%2FPackaging-market-and-challenges-in-2016&usg=AOvVaw1NRZNaZACeCTU88su2U2wZ (accessed on 30 March 2023).
- Phelan, A.A.; Meissner, K.; Humphrey Ross, H. Plastic pollution and packaging: Corporate commitments and actions from the food and beverage sector. J. Clean. Prod. 2022, 331, 129827.
- Ketelsen, M.; Janssen, M.; Hamm, U. Consumers’ response to environmentally-friendly food packaging—A systematic review. J. Clean. Prod. 2020, 254, 120123.
- Li, Y.; Yan, H.; Li, X.; Ge, J.; Cheng, J.; Yu, X. Presence, distribution and risk assessment of phthalic acid esters (PAEs) in suburban plastic film pepper-growing greenhouses with different service life. Ecotoxicol. Environ. Saf. 2020, 196, 110551.
- Pervez, S.; Nawaz, M.A.; Jamal, M.; Jan, T.; Maqbool, F.; Shah, I.; Aman, A.; Qader, S.A.U. Improvement of catalytic properties of starch hydrolyzing fungal amyloglucosidase: Utilization of agar-agar as an organic matrix for immobilization. Carbohydr. Res. 2019, 486, 107860.
- Nie, Z.; Peng, K.; Lin, Z.; Yang, J.; Cheng, Z.; Gan, Q.; Chen, Y.; Feng, C. A conductive hydrogel based on nature polymer agar with self-healing ability and stretchability for flexible sensors. Chem. Eng. J. 2023, 454, 139843.
- Huang, D.; Zhang, Z.; Zheng, Y.; Quan, Q.; Wang, W.; Wang, A. Synergistic effect of chitosan and halloysite nanotubes on improving agar film properties. Food Hydrocoll. 2020, 101, 105471.
- Hasija, V.; Sharma, K.; Kumar, V.; Sharma, S.; Sharma, V. Green synthesis of agar/Gum Arabic based superabsorbent as an alternative for irrigation in agriculture. Vacuum 2018, 157, 458–464.
- Kavoosi, G.; Derakhshan, M.; Salehi, M.; Rahmati, L. Microencapsulation of zataria essential oil in agar, alginate and carrageenan. Innov. Food Sci. Emerg. Technol. 2018, 45, 418–425.
- Jridi, M.; Abdelhedi, O.; Zouari, N.; Fakhfakh, N.; Nasri, M. Development and characterization of grey triggerfish gelatin/agar bilayer and blend films containing vine leaves bioactive compounds. Food Hydrocoll. 2019, 89, 370–378.
- Wongphan, P.; Harnkarnsujarit, N. Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films. Int. J. Biol. Macromol. 2020, 156, 80–93.
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N. Comparative study on antimicrobial activity and biocompatibility of N-selective chitosan derivatives. React. Funct. Polym. 2018, 124, 149–155.
- Oladzadabbasabadi, N.; Mohammadi, A.; Nafchi, F.; Ariffin, M.M.; Wijekoon, J.O.; Al-Hassan, A.A.; Dheyab, M.A.; Guasemlou, M. Recent advances in extraction, modification, and application of chitosan in packaging industry. Carbohydr. Polym. 2022, 277, 118876.
- Kaushalya, K.G.D.; Gunathilake, K.D.P.P. Encapsulation of phlorotannins from edible brown seaweed in chitosan: Effect of fortification on bioactivity and stability in functional foods. Food Chem. 2022, 377, 132012.
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 23, 85–98.
- Cheba, B.A. Chitosan: Properties, Modifications and Food Nanobiotechnology. Procedia Manuf. 2020, 46, 652–658.
- Khan, A.; Wang, B.; Ni, Y. Chitosan-Nanocellulose Composites for Regenerative Medicine Applications. Curr. Med. Chem. 2020, 28, 4584–4592.
- Tzaneva, D.; Simitchiev, A.; Petkova, N.; Nenov, V.; Stoyanova, A.; Denev, P. Synthesis of Carboxymethyl Chitosan and its Rheological Behaviour in Pharmaceutical and Cosmetic Emulsions. J. Appl. Pharm. Sci. 2017, 7, 70–78.
- Bakashi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083.
- Badawy, M.E.I. Structure and antimicrobial activity relationship of quaternary N-alkyl chitosan derivatives against some plant pathogens. J. Appl. Polym. Sci. 2010, 117, 960–969.
- Ghaderi, J.; Hosseini, S.F.; Keyvani, N.; Gómez-Guillén, M.C. Polymer blending effects on the physicochemical and structural features of the chitosan/poly(vinyl alcohol)/fish gelatin ternary biodegradable films. Food Hydrocoll. 2019, 95, 122–132.
- Mendes, J.F.; Paschoalin, R.; Carmona, V.; Neto, A.R.S.; Marques, A.; Marconcini, J.; Mattoso, L.; Medeiros, E.; Oliveira, J. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458.
- Rocha, M.; Alemán, A.; Romani, V.P.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Effects of agar films incorporated with fish protein hydrolysate or clove essential oil on flounder (Paralichthys orbignyanus) fillets shelf-life. Food Hydrocoll. 2018, 81, 351–363.
- Contessa, C.R.; Rosa, G.S.; Moraes, C.C. New Active Packaging Based on Biopolymeric Mixture Added with Bacteriocin as Active Compoun. Int. J. Mol. Sci. 2021, 22, 10628.
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492.
- Fathiraja, P.; Gopalrajan, S.; Karunanithi, M.; Nagarajan, M.; Obaiah, M.C.; Durairajm, S.; Neethirajan, N.V. Development of a biodegradable composite film from chitosan, agar and glycerol based on optimization process by response surface methodology. Cellul. Chem. Technol. 2021, 55, 849–865.
- Cao, Q.; Zhang, Y.; Chen, W.; Meng, X.; Liu, B. Hydrophobicity and physicochemical properties of agarose film as affected by chitosan addition. Int. J. Biol. Macromol. 2018, 106, 1307–1313.
