1. Liposomes: Versatile Drug Delivery Systems
Antibiotics can be delivered to biofilms via conjugation to, or encapsulation within, the nano-drug delivery system. Encapsulation is particularly beneficial as it shelters the enclosed agent from potential inactivation and degradation, and reduces its associated toxicity and side effects. Liposomes are one of the most promising and commonly studied nano-vehicles to carry and deliver antimicrobial agents. This is mainly due to their ability to carry a wide range of antibiotics and infiltrate the extra polymeric matrix
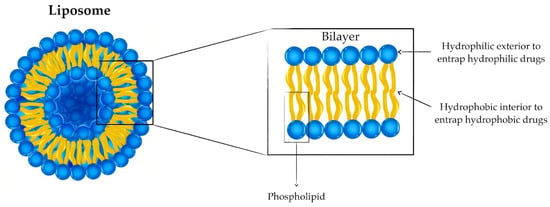
[1][15]. Liposomes are spherical vesicles of diameters typically between 50 and 500 nm with a hydrophilic interior formed by the self-assembly of lipids into a lipid bilayer. Liposomes can carry hydrophilic and hydrophobic cargo within their inner cavity and the bilayer itself, respectively, as shown in
Figure 1 [1][2][3][8,15,33].
Figure 1.
Structure of liposomes to entrap hydrophilic and hydrophobic drugs.
Liposomes can be formulated from a wide range of amphiphilic lipids, including phospholipids and glycolipids, which determine the properties of the liposome. While the surface properties of liposomes are determined by the lipid’s polar heads, the fluidity of the membrane is dictated by the nonpolar portion of the lipid. A major advantage of liposomes for delivery purposes is their similarity to biological cell membranes. In antimicrobial applications, this advantage facilitates the fusion of the liposome to the bacterial membrane, allowing the delivery of the antibiotic into the cytoplasm of the bacteria
[1][2][4][8,15,34].
Due to their advantageous properties, different types of liposomes have been explored to perforate bacterial biofilms, improve antibiotic delivery, reverse antibiotic resistance, and inhibit growth in an array of gram-positive and -negative strains
[5][6][7][8][9][10][35,36,37,38,39,40]. The liposomal types investigated for biofilm treatment can be classified according to composition into conventional first-generation liposomes (neutral, cationic, or anionic), stealth (PEGylated) liposomes, actively targeted liposomes, stimuli-responsive liposomes, and bubble liposomes
[3][11][33,41]. In addition, liposomes can also be classified based on their size and number of bilayers into unilamellar vesicles (small and large), multilamellar vesicles, and oligolamellar vesicles
[12][42]—the different types of liposomes and their advantages and disadvantages as delivery systems are discussed below.
Conventional liposomes are non-modified liposomes in which the lipid bilayer comprises cationic, anionic, or neutral phospholipids together with cholesterol (
Figure 1). Although proving advantageous in reducing the toxicity of the liposome-encapsulated drug, conventional liposomes as therapeutic drug carriers are limited by their facile clearance from the bloodstream by macrophages of the reticuloendothelial system post-opsonization
[11][12][41,42]. This elimination is more pronounced for cationic and larger liposomes due to the electrostatic interaction between the positively charged liposomes and the negatively charged biological macromolecules
[12][13][42,43]. In terms of antibacterial and anti-biofilm activities, cationic liposomes tend to be more effective compared to negative or neutral liposomes of the same size
[4][34]. The cationic antimicrobial particles may reduce bacterial adhesions, thereby disrupting the formation of the biofilm
[14][44]. This is possibly due to the electrostatic attractive forces between the positively charged liposomes and the negative surface of the bacteria/biofilm
[1][15][15,45]. Furthermore, first-generation liposomes lack any active targeting mechanisms and utilize only passive targeting to localize the vehicle within tissues having a discontinuous endothelial lining
[12][42].
Despite their advantages, the efficiency of conventional liposomes is limited for applications involving skin penetration due to their rigidity. Therefore, deformable (or elastic) liposomes have been developed to enhance drug delivery across the skin membrane. Due to their higher elasticity that surpasses that of conventional liposomes, deformable liposomes are more suitable for skin penetration into deeper layers of the epidermis
[16][17][46,47].
Furthermore, to prolong their circulation time, sterically stabilized liposomes with longer blood circulation time and protection from macrophages were developed. One of the most commonly studied stabilized liposomes are polyethylene glycol (PEG) liposomes, also called stealth liposomes. PEG is a non-ionic hydrophilic polymer commonly used on the surfaces of drug delivery systems to mask them from opsonization and hence, elimination by the reticuloendothelial system. Furthermore, PEG coating prevents aggregation of the liposomes via steric stabilization
[12][18][42,48]. PEGylated liposomes have been extensively reported as superior alternatives to conventional liposomes in terms of blood circulation
[19][20][21][22][23][49,50,51,52,53]. However, stealth liposomes suffer from disadvantages, including their potential hypersensitivity (which depends on several parameters such as size) and their non-biodegradability, which restricts their use to low molecular weight PEGs
[18][48].
To solve the issue of the non-specificity of conventional liposomes, actively targeted liposomes have been established in which the liposome surface is modified with a moiety, such as antibodies that can target a specific tissue or organ in the body. For instance, immunoliposomes (antibody-modified liposomes) have been commonly reported for efficient antibody-mediated targeting for applications including biofilm treatment
[24][25][26][27][28][29][54,55,56,57,58,59]. However, bare immunoliposomes without steric stabilization are inefficient due to their rapid elimination. Hence, immunoliposomes are usually modified with PEG to achieve targeting while avoiding rapid clearance from circulation
[24][54]. Other ligands used to target liposomes include aptamers
[30][31][60,61] and peptides
[32][62].
Stimuli-responsive liposomes are another type of liposome attracting extensive attention due to their ability to respond to certain stimuli and achieve a controlled and targeted drug release. Exploiting stimuli for targeted drug release is especially beneficial as it reduces the risk of off-target drug toxicity
[33][34][63,64]. Such stimuli responsiveness can be achieved by modifying the lipids making up the bilayer to respond to endogenous or external stimuli
[13][43]. Endogenous (or internal) stimuli include internal conditions and triggers such as redox conditions or pH, while exogenous (or external) stimuli include externally applied triggers such as light and ultrasound
[33][63]. Although endogenous stimuli have been reported with other nanoparticles, none have been used with liposomes. As for exogenous stimuli, the biofilm microenvironment differs from normal tissue environments in its low pH, elevated H
2O
2 levels, overexpression of some enzymes, and accumulation of the thiol glutathione (GSH). The presence of such distinct environmental features within the biofilm makes it possible to use those internal conditions as triggers for the biofilm-specific release of anti-biofilm agents
[35][65]. To benefit from exogenous stimuli, liposomes have been combined with other nanoparticles that can respond to externally applied triggers. For example, thermo-sensitive liposomes have been combined with gold nanoparticles which respond to near-infrared light by generating heat. The generated heat causes the liposome to undergo a phase transition and become more fluidic, thereby releasing encapsulated cargo
[36][66]. Exogenous stimuli, such as near-infrared irradiation, have been reported to trigger drug release from liposomes for biofilm treatment, as discussed in the upcoming section of this review
[37][38][39][67,68,69].
Bubble liposomes utilize ultrasound to burst a gas bubble encapsulated within the liposomes together with the drug(s) to be delivered. Bursting of the bubble post-ultrasound application disrupts the liposome and leads to the controlled release of hosted drug(s)
[40][70]. However, bubble liposomes are challenged by their low gas loading capacity and large sizes (from 500 nm to microns), and their costly and complicated preparation procedures
[40][70]. Bubble liposomes have been explored for biofilm treatment, as reported by Fu, et al.
[41][71] and Zhou, et al.
[42][72].
Despite the advantages provided by each liposomal formulation, they still possess their own limitations. For instance, while conventional liposomes reduce drug-associated toxicities, they still suffer from their rapid removal from circulation
[11][41]. This issue is solved by the introduction of PEG on the surface of liposomes. However, PEG coating is limited by the non-biodegradability of high-molar-mass PEG and the toxicity of low-molar-mass PEG. Therefore, it is important to ensure that the molar mass of PEG does not surpass the limit of renal elimination. Further adding to the issue is the difficulty in determining PEG’s threshold of renal elimination
[18][48]. Due to their rigidity, conventional liposomes also face a challenge in penetrating the skin barrier in the case of topical applications. This issue is overcome using elastic (deformable) liposomes, which have better skin penetration due to their higher elasticity
[17][47]. Nevertheless, deformable liposomes also suffer from limitations due to their prolonged elasticity and deformability; thus, these liposomes are typically unstable over extended storage periods. This, in turn, results in the loss of the liposome content during the storage period, which in turn impedes upscaling of the formulation
[17][47]. Like conventional liposomes, targeted liposomes also suffer from rapid elimination from the bloodstream unless sterically stabilized (e.g., by PEG)
[24][54].
On the other hand, non-specific release by liposomes can limit their therapeutic efficacy by reducing their bioavailability at the target site. To overcome such non-specificity, stimuli-responsive liposomes, which can release their cargo in response to internal and/or external stimuli, can be used
[13][33][34][43,63,64]. However, the limitations of stimuli-responsive liposomes vary depending on the type of stimulus used. For instance, ultraviolet-responsive liposomes are limited due to ultraviolet’s poor tissue penetration and damage to cells/tissues
[43][73]. Furthermore, it is important for the liposomes to have a high drug encapsulation efficiency, which can improve the bioavailability of the drug. However, encapsulation efficiency is dependent on the type of phospholipids making up the liposomes
[44][74]. Therefore, it is important to ensure liposomal formulations for drug delivery, including antibiofilm agent delivery, are stable, have high encapsulation efficiency, are not rapidly eliminated from the bloodstream, and have high bioavailability (e.g., by targeting and stimuli-responsivity). The different types of liposomes are summarized in
Table 1.
While other nano-drug delivery vehicles, such as metallic and polymeric nanoparticles, have been developed and studied, liposomes are particularly advantageous. This is due to their ability to carry and deliver hydrophilic and/or hydrophobic cargo, high biocompatibility, biodegradability, lack of immunogenicity and toxicity, and easy modification with targeting moieties. Furthermore, several liposome-based compositions have been approved by the FDA for the clinical treatment of infectious diseases. This indicates the promise of future liposomal formulations
[45][13]. However, as mentioned in the previous paragraph and
Table 1, liposomes do possess some limitations that need to be considered when designing a liposomal formulation for drug delivery purposes, including anti-biofilm agent delivery.
2. Liposomal Formulations for the Treatment of Gram-Negative Biofilms
Prominent agents behind global mortality and morbidity, gram-negative bacteria are less susceptible to antibiotic agents than gram-positive bacteria
[46][75]. Unlike their gram-positive counterparts, gram-negative bacteria, except for the lipopolysaccharide (LPS)-deficient strains, are enveloped with LPS
[47][76]. LPS serves as a stimulator of the immune system and a facilitator of antibiotic resistance due to its insulation of the bacterial cell it encapsulates
[48][77]. In fact, LPS-deficient strains are less virulent and more susceptible to antibiotics
[47][76]. Accordingly, it is no surprise that gram-negative bacteria constitute the majority of the WHO antibiotic-resistant pathogens list
[46][48][75,77]. Some of the prominent gram-negative strains include
P. aeruginosa,
E. coli,
Acinetobacter baumannii (
A. baumannii),
Salmonella,
Klebsiella,
Serratia,
Aeromonas, and
Porphyromonas spp. The following sections summarize recent studies implementing liposomal formulations against medically salient gram-negative bacteria.
3. Liposomal Formulations for the Treatment of Gram-Positive Biofilms
As with gram-negative bacteria, infections due to gram-positive biofilms present a major health challenge, especially due to the emerging resistance of gram-positive strains to antibiotics
[49][50][116,117]. Gram-positive bacteria are responsible for the majority of infections in intensive care units in hospitals and are the leading cause of skin, soft tissue, and medical device-associated infections
[51][52][118,119]. Further adding to the urgency of gram-positive medical infections is their susceptibility to form biofilms, which are generally responsible for the vast majority of clinical chronic infection cases
[53][54][120,121]. Several types of clinically relevant gram-positive strains that have the tendency to develop biofilms include the
Staphylococcus strains:
S. aureus, including
Methicillin-resistant S. aureus (MRSA) and
Methicillin-susceptible Staphylococcus aureus (MSSA);
S. epidermidis; and
Staphylococcus saprophyticus subspecies Bovis, as well as other gram-positive strains, such as
Listeria,
Mycobacterium,
Cutibacterium acnes (
C. acnes), and
Bacillus subtilis. Various liposomal composites have been developed in efforts to overcome the resistance and treat biofilms of those bacterial strains. The subsequent sections discuss the progress in the use of liposomal formulations for the treatment of gram-positive biofilms. Specific focus is given to therapeutic parameters, including triggered release, biofilm targeting mechanisms, loaded drugs, including antibiotics and their alternatives, and the efficiency of the composite at eradicating the biofilm in vitro and in vivo.