There are significant gender differences in the relationship between cortisol and depression. While the results may vary based on the age group and source of the samples, several mixed-gender studies show that there appears to be heightened serum cortisol levels in depressed males compared to depressed females. There are also differences noticed in salivary cortisol reactivity, with male children having increased reactivity, though these differences seem to diminish with age. Significant gender differences can also be seen in glucocorticoid and mineralocorticoid receptor expression. These differences are found both in expression levels as well as epigenetic regulation depending on the disorder and on the brain region.
1. Introduction
One of the important biomarkers in clinical studies for stress and depression is cortisol, a glucocorticoid hormone regulated by the hypothalamic-pituitary-adrenal (HPA) axis that is postulated to play an important role in mental health outcomes, particularly depression
[1][6]. In a healthy and stress-free individual, cortisol is released according to the circadian rhythm and can be divided into two phases: the cortisol awakening response (CAR) and the diurnal cortisol slope (DCS). The first phase, CAR, involves a rapid increase in cortisol levels upon a person waking up, generally reaching peak levels after 30 to 45 min. The second phase, DCS, follows with a gradual decrease in cortisol levels throughout the rest of the day
[2][7]. Any imbalances or changes in the pattern of cortisol release can become a contributing factor to negative mental health outcomes including depression.
Though depression is a complex disease that can have many causes, females are known to be at higher risk of being diagnosed with depression compared to males, which is a phenomenon that could be attributed to the exposure of chronic stressors as well as the effect of gonadal hormones
[3][8]. Females experience more hormonal changes during their lifetime through puberty, menstruation, pregnancy, and menopause, which could make them more susceptible to mental illnesses such as depression
[4][9]. However, while there has been research regarding gender differences in stress and depression, few studies have set out to specifically investigate the difference in the release of cortisol between females and males in relation to depression.
Glucocorticoids have been known to play an important role in mental health for a long time. Released during stress, glucocorticoids have been associated with depression and mood disorders
[5][10]. It stands to reason that the receptors for these glucocorticoids are also important subjects of study. Glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs) are the target receptors in the hypothalamic-pituitary-adrenal (HPA) axis during stress
[6][11]. Increasing evidence suggests that GRs and MRs have different affinities and binding activities to cortisol
[7][12]. Both receptors show different reactions to cortisol and functionally interact with each other during stress in the HPA axis seen in clinical and animal studies
[7][12].
While changes in GR and MR functions in association with stress are known to be associated with mental health disorders, more recent research also includes gender differences in the HPA’s response to stress
[8][13]. GRs and MRs have different effects on mood control and the related behavior in males and females
[9][14]. What works as a treatment for one gender may not be as effective for the other. There is also increasing evidence that GRs and MRs play a key role in the functionality of the HPA axis and determine susceptibility to stress-related mental disorders
[9][14]. These findings suggest that GRs and MRs have a sex-specific influence on the relationship between stress responses in the brain and stress-related mental disorders such as depression. The sex-specific functions and stress reactions of GRs and MRs in the etiology of depression remain relatively unexplored. As such, the roles of GRs and MRs in mental health should be re-examined in a gender-based context. The existence of a genetic risk of GRs and MRs associated with stressful conditions or the recurrence of stress-related mental disorders has been posited. In particular, there is evidence to support the association of various common single nucleotide polymorphisms of GR and MR genes and their derived haplotypes with sensitivity to cortisol, as well as the onset and presence of major depression.
2. Structure and Function of Glucocorticoid and Mineralocorticoid Receptors in the HPA Axis
The human glucocorticoid receptor (GR) is coded by the NR3C1 gene, which is located on chromosome 5q31.3, and consists of three main structural and functional domains
[6][10][11,21]. The human NR3C1 gene consists of 10 exons. Of these exons, the first, exon 1, is an untranslated region (UTR). Meanwhile, the N-terminal modulatory domain (NTD) is encoded by exon 2, while exons 3 and 4 encode the DNA binding domain (DBD). Finally, the hinge region (HR) and ligand-binding domain (LBD) are encoded by exons 5 to 9
[6][11]. In contrast to other steroid hormone receptors such as progesterone and estrogen, there is no F region to be found in the GR. From the NR3C1 gene, the classic GR isoform as well as the non-ligand-binding GR isoform are produced from the alternative splicing of two terminal exon 9s
[11][22]. The NTD is where transcriptional activation function 1 (AF1) is located and serves as the site for most post-translational modifications
[12][23]. The DBD contains two zinc finger motifs that bind to their target DNA sequences, glucocorticoid responsive elements (GREs)
[12][23]. The LBD is where glucocorticoids bind and contains the transcriptional activation function 2 (AF2) that engages in ligand-dependent interactions with co-regulators
[12][23]. GRs can be found in relatively high quantities throughout the human brain
[13][24], with higher concentrations found in the frontal area, hypothalamus, hippocampus
[14][25], and amygdala
[15][26].
The mineralocorticoid receptor (MR) is coded for by the NR3C2 gene, which is located on chromosome 4q31. While the MR is structurally and functionally similar to the GR, differences in the LBD lead to the MR having a higher affinity for ligands such as aldosterone
[16][17][27,28]. MRs are found throughout the human brain, though they are mainly localized in the limbic region, such as the hippocampus and the dorsolateral septum
[18][29].
There have been more discoveries in recent years about the mechanisms of GRs and MRs in relation to HPA activity. Increased microRNA-124 (miR-124) levels have been associated with major depressive disorder
[19][34]. MiR-124 directly targets GRs and when inhibited alleviates depressive-like symptoms in mice
[20][35]. Furthermore, hypermethylation of NR3C1 exon 1F, which is related to higher basal HPA activity, has been observed in depressed patients who had experienced early life stress
[21][36]. Genomic analyses reveal that differences in haplotypes for N3C1 and N3C2 affects stress-induced reactivity to cortisol, which subsequently impacts cognitive behavior
[22][37]. Chaperone proteins such as Hsps and FKBPs have also been implicated in major depressive disorders, as certain SNPs for Hsps and FKBPs have been linked to a higher incidence of the disease
[23][24][38,39], while increased FKBP51 expression in particular has been observed in depressed patients
[25][40]. The role of the GR/MR in the HPA axis under depression is summarized in
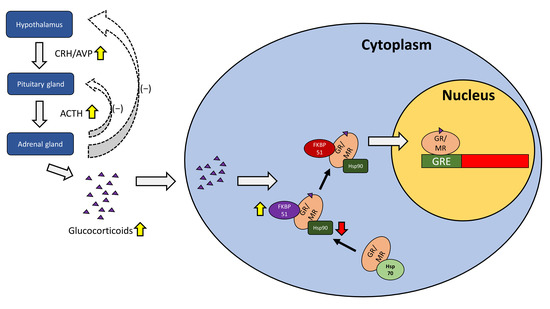
Figure 1.
Figure 1. The role of the GR/MR in the HPA axis under depression. The hypothalamus releases corticotropin-releasing hormone (CRH) and arginine-vasopressin (AVP), acting on the pituitary gland which in turn releases adrenocorticotropic hormone (ACTH) to stimulate the adrenal gland. The adrenal gland plays a role in the negative feedback loop for the hypothalamus and the pituitary gland, while releasing glucocorticoids such as cortisol. Glucocorticoids travel into the cell and bind to GRs or MRs, with the help of Hsp90 and FKBP51, before being transported into the nucleus with the aid of FKBP52 where they activate GREs for subsequent gene transcription. During depression, CRH and AVP are elevated, resulting in increased ACTH and increased glucocorticoid release such as cortisol. Chaperone proteins such as FKBP51 may be elevated while others such as Hsp90 may be impaired in function.
3. Gender Differences in Glucocorticoid and Mineralocorticoid Receptors in Mental Health
3.1. Gene Expression of Glucocorticoid Receptor- and Mineralocorticoid Receptor-Related Genes
Genetic analysis of GR-related genes revealed that a polymorphism of the NR3C1 GR gene is significantly associated with major depression in women
[26][63]. The frequency of the polymorphism was more than three times higher in women suffering from depression
[26][63]. The same study did not find NR3C2 MR gene polymorphisms to be significantly associated with depression, nor did it discover any male-associated polymorphisms linked to depression
[26][63].
Examination of specific MR haplotypes showed that haplotype CA is associated with enhanced MR activity and increased behavioral optimism
[27][65]. This haplotype is also linked to a lower risk of depression when examining data from a large genome-wide association study, although the correlations are valid only in women
[27][65]. More studies investigating MR haplotypes discovered other haplotypes that influence susceptibility to depression following childhood maltreatment
[28][66].
Besides depression, GRs and MRs have also been associated with other mental health disorders such as schizophrenia and psychosis. Thus far, there has been little to suggest that gender differences in GR expression are present in schizophrenia and other mood disorders. Examination of GR mRNA levels in post-mortem human brain specimens suffering from bipolar disorder and schizophrenia found evidence of GR dysregulation in varying regions of the brain depending on the disorder but no significant gender differences
[29][68]. The same appears to hold true for MR studies.
3.2. Gender Differences in Animal Studies of Glucocorticoid and Mineralocorticoid Receptor Expression
The presence of gender differences in GR/MR expression in early-life-stress-induced depression appears to be supported by an early life stress animal model. Reactivity of the HPA axis was reduced in female mice exposed to early life stress and further exacerbated by decreased MR expression
[30][73]. Male mice showed an increase in HPA reactivity as well as elevated upregulation of MR
[30][73]. Gender differences in GR and MR expression are also seen in avian species. Social stress via mate pair separation of zebra finches revealed that female finches displayed an increase in hippocampal MR but not GR, while males showed a decrease in both hippocampal MR and GR
[31][74].
A study performed on socially isolated female rats observed an increase in depressive-like behavior that subsides during estrus along with a reduction in GR expression in the hippocampus
[32][75]. Another experiment performed on juvenile male rats showed GR expression increased and MR expression decreased during social isolation
[33][76]. Furthermore, stress increased GR expression in group-housed rats with time, while it did not affect GR expression in isolated rats
[33][76]. These studies indicate the influence of social isolation on altering GR expression. Furthermore, the finding that ovarian hormones may reduce GR expression
[32][75] suggests a gender difference present in how GR activity is affected by social isolation.