Tight junctions (TJs) are multiprotein complexes present at the tip of the lateral membrane of polarized epithelial and endothelial cells
[1]. The main function of these structures is to mediate adhesion and polarity between cells. Therefore, it is believed that the impairment of TJs and the concomitant loss of cell-to-cell adhesion are necessary for the early stages of cancer invasion and metastasis. However, it is becoming increasingly clear that TJ proteins such as claudins play important roles not only in adhesion but also in the activation of intracellular signaling, which contributes to tumor progression and metastasis. Claudin (CLDN) is a cell-to-cell adhesion component of the tight junctions and forms a protein family with 27 highly homologous members
[2][3][2,3]. CLDN4 is the major CLDN involved in TJs in epithelial cells, such as those in the intestines and lungs, and is associated with many epithelial malignancies
[4][5][4,5].
2. CLDN4 Expression and Regulation in Cancer
Overexpression of CLDN4 has been reported in various cancers, such as gastric cancer
[6][7][8][6,7,8], pancreatic cancer
[9][10][11][9,10,11], colorectal cancer
[12][13][12,13], breast cancer
[14][15][14,15] (especially triple-negative breast cancer
[16][17][16,17]), oral squamous cell carcinoma
[18], ovarian cancer
[19], bladder cancer
[20][21][20,21], non-small cell lung cancer
[22], and cholangiocarcinoma
[23]. In all of these cases, CLDN4 expression correlated with disease progression and poor prognosis. CLDN4 is also overexpressed in thyroid cancer
[24] and prostate cancer
[25][26][25,26]; however, in these cancers, decreased expression correlated with poor prognosis.
2.1. Epigenetics
Epigenetic alterations play a major role in carcinogenesis and cancer progression in various malignancies
[27][28][29][29,30,31]. Changes in DNA methylation, histone modifications, chromatin remodeling, and microRNAs are considered useful indicators of cancer development and progression
[27][29], and epigenetic changes in the regulation of CLDN4 expression have recently been reported. Hypermethylation of CpG islands in the
CLDN4 promoter region reduces CLDN4 expression in gastric, bladder, and colon cancers
[30][31][32][32,33,34]. In contrast,
CLDN4 hypomethylation and CLDN4 overexpression have been reported in gastric, breast, ovarian, and bladder cancers
[21][33][34][35][21,35,36,37].
Epigenetic regulation of CLDN expression has also been reported for CLDN1
[32][36][37][34,41,42], CLDN2
[38][43], CLDN3
[30][39][40][32,44,45], CLDN6 and CLDN9
[41][46], CLDN7
[42][47], and CLDN11
[43][48]. Several studies have also indicated the involvement of microRNAs in the regulation of CLDN4 expression.
CLDN4 is a target gene of miR497-3p and the long non-coding RNA ELFN1-AS1, which promotes
CLDN4 expression by sponging miR497-3p
[44][49].
2.2. Inflammatory Processes
In gastric cancer, CLDN4 expression is elevated in
Helicobacter pylori-positive cases
[8]. Here, CLDN4 expression is upregulated by CDX2, leading to an intestinal phenotype induced by
H. pylori infection
[45][51]. CLDN4 is downregulated by inflammatory cytokines such as TNFα and HMGB1
[13][46][13,52]. In rheumatoid arthritis, blood IL-4, -5, -6, and -13 levels are elevated, while the levels of CLDN4, 7, 12, and 15, as well as ZO-1, are decreased
[47][53]. IL-18 represses the expression of CLDN1, 3, 4, and 12
[48][54].
2.3. Growth Factors
Smad signaling triggered by TGF-β induces CLDN4 promoter activity via c-Jun, enhancing CLDN4 expression
[49][60]. In mouse intestinal epithelium, knockdown of smad4 has been shown to reduce the expression of CLDN3 and 4, but increase that of CLDN2 and 8, resulting in increased intestinal permeability
[50][61]. In glioblastoma, TGFβ promotes CLDN4 expression and enhances invasive ability
[51][62]. Other signaling pathways that affect CLDN4 expression include PKCα
[52][63], twist
[53][64], ERK1/2
[54][65], p38MAPK
[55][66], HIF1α
[56][67], and hedgehog
[57][68].
3. The Function of CLDN4 in Cancer
3.1. Carcinogenesis
CLDN4 overexpression has been detected in several cancers, including lung, gastric, colorectal, endometrial, uterine cervical, and ovarian epithelial cancers. In these cancers, precancerous lesions, atypical adenomatous hyperplasia, gastric dysplasia, sessile serrated adenoma/polyp with dysplasia (SSA/P-D), atypical endometrial hyperplasia, cervical intraepithelial neoplasia (CIN), and borderline malignant lesions display increased expression and/or abnormal distribution of CLDN4
[58][59][60][61][62][63][69,70,71,72,73,74].
3.2. Barrier Function and Maintenance of Intratumoral Microenvironment
CLDN4 is a major structural protein of epithelial TJs in intestines and lungs and is involved in epithelial differentiation, polarity maintenance, and substance trafficking
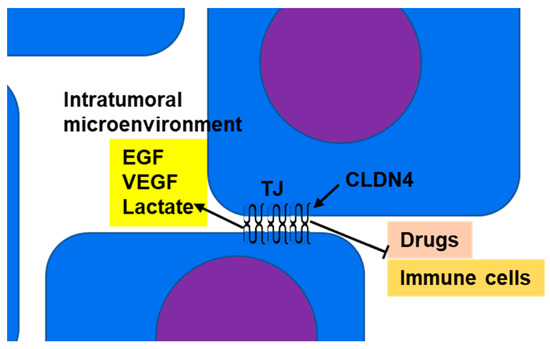
[64][65][80,81]. In normal epithelial tissue, TJs act as barriers or gates that separate the outside from the inside of the body and restrict material transport; however, in tumor tissue, the polarity of the cells and tissues is ambiguous. Thus, in CLDN4-overexpressing epithelial malignancies, the barrier function of TJs serves to maintain the tumor microenvironment and retain tumor-secreted growth factors to promote the malignant cancer phenotype (
Figure 1)
[8][11][13][16][20][8,11,13,16,20].
Figure 1. Barrier function of TJs: CLDN4, which is overexpressed in epithelial malignancies, separates the intratumoral microenvironment from the tumor exterior by forming TJs. As a result, growth factors (such as EGF and VEGF) and metabolites (such as lactate) accumulate in the intratumoral microenvironment, resulting in amplification of their effects. This promotes increased tumor grade and suppression of immune cell infiltration into the tumor. TJs also inhibit the penetration of anticancer drugs from the tumor exterior into the microenvironment and enhance resistance to anticancer drugs. CLDN4, claudin-4; TJ, tight junction; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor.
In assessing the barrier action of TJ CLDN4, it is necessary to consider its involvement in transport in normal TJs. TJ controls material transport through the paracellular and transcellular routes. Utilization of these pathways is affected by the ratio of ionized and unionized species (which depends on the pKa of the drug, the size of the molecules, and the pH of the solution), the intrinsic partition coefficient of the drug, and the size of the molecule and its charge
[66][86].
3.3. Apoptosis
Several studies have suggested that CLDN4 expression is involved in promoting cancer cell viability. CLDN4 suppresses cell death caused by apoptosis
[15][67][15,89] and endoplasmic reticulum stress
[68][90]. It is believed that non-TJ CLDN4 activates integrin β1 as a binding partner and suppresses apoptosis via FAK signaling
[8][21][8,21]. In addition, CLDN1
[69][70][91,92] increases resistance to anoikis, whereas CLDN6 decreases it
[71][93]. However, the effect of CLDN4 on anoikis is still unclear.
3.4. Stemness and EMT
In EMT, epithelial cells lose epithelial differentiation and transition to mesenchyme, which involves background dedifferentiation from the epithelium and enhanced stemness
[72][94]. It has become clear that cancer stemness is the basis of tumorigenicity, self-renewal, and differentiation, as well as tumor heterogeneity, metastasis, and treatment resistance
[73][95]. Decreased expression of CLDN4, an epithelial marker in tumors, reflects EMT and is also associated with metastasis
[74][75][96,97]. EMT correlates with hypermethylation of the
CLDN4 promoter, which causes downregulation of CLDN4
[76][98], while the transcription factor Bach1 directly suppresses the expression of
CLDN4 and
E-cadherin to induce EMT
[77][99]. Thus, CLDN4 expression is a marker of epithelial traits, and its decrease is thought to be suggestive of EMT. However, Ma et al. reported that repression of CLDN4 expression suppresses invasion and metastasis in breast cancer cell lines
[15].
4. Non-TJ Functions of CLDN4
4.1. Non-TJ Plasma Membrane CLDN4
CLDN4 is overexpressed in bladder cancer due to promoter DNA hypomethylation
[21]. Further demethylation via aza-2′-deoxycytidine (AZA) treatment induces expression of CLDN4 to levels above that necessary for TJ function. This is accompanied by the formation of CLDN4 monomers that do not incorporate into TJs
[21]. This process is considered to be one of the mechanisms responsible for generating non-TJ CLDN4.
4.2. Cytoplasmic CLDN4
CLDN4 can also be taken from TJs to form a non-plasma membrane (cytoplasmic) CLDN4. Studies have demonstrated that the C-terminus domain of
Clostridium perfringens enterotoxin (CPE) binds to the second extracellular loop of CLDN4, disrupting homotypic claudin binding, impairing TJs, and leading to diarrhea
[78][104]. As a result, CLDN4 is released from TJs and translocated into the cytoplasm
[13][18][13,18].
4.3. Function of Non-TJ CLDN4
4.3.1. Integrin β1 Activation
Integrin β1 activates FAK and induces the expression of stem cell-related genes such as Oct4, Sox2, and Nanog through Notch signaling
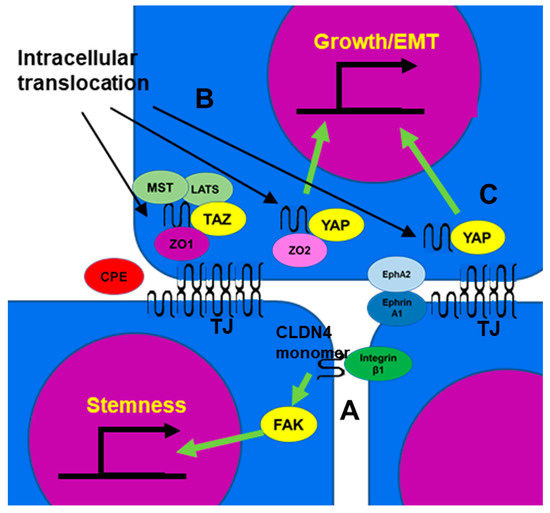
[79][80][105,106]. Non-TJ CLDN4 binds to integrin β1 and enhances stemness, anti-apoptotic effects, drug resistance, and metastatic capacity of cancer cells (
Figure 2A)
[8][21][8,21]. In poorly differentiated gastric cancer, TJ formation is reduced, but EMT is mediated by non-TJ CLDN4
[8]. CLDN7, like CLDN4, also binds to integrin β1, leading to downstream FAK phosphorylation
[81][82][107,108]. CLDN4 exhibits approximately 40% of the affinity of CLDN7 for integrin β1
[8].
Figure 2. The function of non-TJ CLDN4. (A) CLDN4 monomer that does not form TJs is a binding partner for integrin β1 expressed in neighboring tumor cells, activates FAK, and promotes expression of stemness-associated genes. (B) CLDN4 translocates into the cytoplasm from TJs disrupted by CPE and forms a stable complex with TAZ, MST, LATS, and ZO1, but YAP is released from the complex and translocates into the nucleus with ZO2 to induce expression of YAP target genes, leading to proliferation and EMT. (C) EphA2 activated by Ephrin A1 expressed on the surface of neighboring cells phosphorylates CLDN4 and releases it from TJs. The released CLDN4 is translocated into the nucleus with YAP. CLDN4, claudin-4; TJ, tight junction; CPE,
Clostridium perfringens enterotoxin; TAZ, tafazzin; YAP, yes-associated protein; ZO, zonula occludens; MST, mammalian Ste20-like kinase; LATS, large tumor suppressor kinase; Eph A2, ephrin type-A receptor 2; FAK, focal adhesion kinase; EMT, epithelial-mesenchymal transition.
4.3.2. YAP Activation
Cytoplasmic CLDN4 is also involved in YAP activation
[18][60][83][18,27,71]. CLDN4 translocated to the cytoplasm by
C. perfringens CPE in the intestinal flora forms a stable complex involving TAZ, LATS, MST of HIPPO inhibitory system, and ZO-1. This sequestration of the YAP co-activator TAZ leaves YAP free to bind to ZO-2 and translocate to the nucleus, where it promotes the expression of target genes such as
cyclin E and
snail, stimulating proliferation and inducing EMT (
Figure 2B)
[60][71]. As a result, it promotes carcinogenesis of SSA/P-D, a colonic precancerous lesion, and is associated with BRAF mutations in colorectal cancer
[60][71]. As mentioned earlier, in renal cell carcinoma, EphA2/Ephrin A1 and PKCε translocate CLDN4 into the nucleus, with YAP bound and co-translocated alongside CLDN4. As a result, YAP is activated and increases the malignancy of cancer cells (
Figure 2C)
[83][27]. YAP activation by CLDN also occurs with CLDN6 and CLDN18. Several factors are involved in YAP nuclear translocation and activation, including the inhibition of YAP phosphorylation by LATS
[84][109], CLDN6-ZO2-YAP interactions
[85][110], and the binding of CLDN18 with YAP
[86][111], which together lead to a poor prognosis in gastric cancer
[87][112].
4.3.3. Activation of AKT
CLDN4 has also been linked to AKT signaling. CLDN4 has been shown to induce PIK3R3 and MAP2K2 mRNA expression and activate AKT and ERK1/2 in acute myeloid leukemia cells
[88][113]. This results in accelerated proliferation and poor prognosis for this disease. Another study indicated that SPTBN2 cooperates with CLDN4 to stimulate PI3K/AKT activation
[89][114]. Conversely, there is also a report that silencing CLDN4 activates AKT
[90][102]. CLDN4 limits the activity of β-catenin and PI3K and inhibits the phosphorylation and activity of EphA2 by AKT
[91][79].
5. Targeting CLDN4
5.1. Antibodies
To target CLDN4 with antibodies, it is essential to generate an antibody against its extracellular domain, but it is difficult to generate a single CLDN-specific antibody due to the high homology among CLDN family members
[92][115]. The antibodies reported to be established thus far include monoclonal antibodies that recognize the extracellular loops of both CLDN3 and CLDN4 and their antitumor effects have been confirmed both in vitro and in vivo
[93][94][116,117]. Suzuki et al. generated a monoclonal antibody (KM3900) that recognizes CLDN4 extracellular loop 2 and induces antibody-dependent cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) in vitro and inhibited the growth of pancreatic and ovarian tumors in SCID mice in vivo
[95][118].
5.2. Knockdown
Knocking down CLDN4 in gastric cancer and bladder cancer results in a mild decrease in transepithelial electrical resistance (TER), an indicator of TJ function
[8][21][8,21]. For this reason, CLDN4 knockdown only provides limited disruption of the microenvironment and the promotion of anticancer drug permeability by impairing TJs. One possible reason for this is that the knockdown of a single CLDN may result in other CLDNs maintaining TJ function in its place. However, CLDN4 knockdown does reduce non-TJ CLDN4 and thus inhibits stemness
[21].
5.3. CPE and C-Terminus Domain of CPE (C-CPE)
CPE recognizes specific amino acid sequences in the first and second extracellular loops of CLDN4 and CLDN3 and docks via a pocket of the domain at the C-terminus to disrupt TJs. Furthermore, it perforates the plasma membrane to cause cell death due to the intracellular influx of calcium
[78][96][104,122]. Therefore, CPE exhibits cytotoxicity against cancer cells expressing CLDN4. The antitumor effect of CPE has been demonstrated by experiments in prostate cancer
[97][98][123,124], non-small cell lung cancer
[22], pancreatic cancer
[10], gastric cancer
[99][125], and ovarian cancer
[100][101][126,127].
5.4. Peptide
Attempts have also been made to produce specific peptides as CLDN binding agents. Hicks et al. showed that a small peptide that mimics the DFYNP sequence in the second extracellular loop of CLDN4 impairs CLDN4, leading to the induction of apoptosis and suppression of tumor growth
[67][89]. In light of these promising data, further progress is expected in peptide drug discovery to target CLDN4.
5.5. Delivery of Anti-CLDN4 Drugs
In many cases, CLDN4-targeting drugs such as those described above reach the tumor through blood administration. At this time, the formation of tumor blood vessels is important for the effective delivery of molecular-targeted drugs. As mentioned above, the barrier action of CLDN4 leads to the accumulation of angiogenic factors within the tumor microenvironment and may promote angiogenesis.