Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by ZHICHAO GENG.
Polishing is a process of creating a smooth and scratchless surface by using mechanical, chemical, and electrochemical approaches for reducing surface roughness and enhancing the workpiece’s strength. Roughness directly determines the surface functional performance, and it is usually handled in the final process of machining, namely polishing.
- polishing
- roughness
- sub-nanometer
- atomic and close-to-atomic scale
- ACSM
1. Introduction
Polishing is a process of creating a smooth and scratchless surface by using mechanical, chemical, and electrochemical approaches for reducing surface roughness and enhancing the workpiece’s strength [1,2][1][2]. Roughness directly determines the surface functional performance, and it is usually handled in the final process of machining, namely polishing.
The origins of polishing date to the Stone Age. Sandstones were utilized as polished stones as early as 4800~4600 BC [3]. Since then, polishing has evolved through four distinct eras, distinguished by the roughness scale that each polishing approach can achieve:
- (1)
-
Era without roughness standard: telescopes and spectacles were invented during the renaissance. Although there was no standard for roughness at that time, as early as 1634, it was already realized that polishing was not just cleaning the glass, but reducing the roughness, such as the cloth is shaved by the cropper [4]. The manufacture of lenses, prisms, and mirrors laid the groundwork for the development of polishing technology. In the 19th century, the “trial and error” production method is common to fabricate microscopes. Carl Zeiss and Ernst Abbe introduced diffraction limit, Abbe number and measured Abbe error to measurements, which led to a qualitative change in microscope polishing technology [5,6][5][6].
- (2)
-
Sub-micro scale era: after the second industrial revolution, optical theory and measuring technologies have propelled polishing into the era of standardization. The first roughness standard, ASA B46.1, was issued in 1940. Hereafter, roughness down to 9 µin in Ra (0.23 μm) [7] and 30 µin in RMS (0.76 μm) [8] were realized for rubberized seal and cast dental gold alloy, respectively. In this period, µin is a common unit for evaluating roughness and the highest precision of roughness in polishing was considered to be 0.5~5 µin [9]. It should be noted that Ra and Rz were not used as roughness parameters in the mid-twentieth century. Instead, they used have and hmax, where have is similar to Ra and hmax is similar to Rz.
- (3)
-
Nanometre scale era: in the second half of the 20th century, the invention of precision polishing technologies including chemical mechanical polishing (CMP), ion beam figuring (IBF), magnetorheological finishing (MRF), fluid jet polishing (FJP), and bonnet polishing (BP) allowed the roughness achieved by polishing to reach the nanometre scale, achieving 3.4 nm in Ra for diamond [10], 2 nm in Rmax for metal mirror surfaces made from Cu/Al alloys [11], and 1.6 nm in RMS for BK7 [12].
- (4)
-
Atomic and close-to-atomic scale (ACS) era: the maturation of computer numerical control (CNC) technology and the diversification of measurement techniques have led to a quantum jump in polishing technology at the sub-nanometer scale. Roughness values of 0.5 nm in RMS for tungsten [13], 0.5 nm in Ra for silicon nitride [14], and 0.15 nm in RMS for polysilicon [15] were realized. The sub-nanometer roughness indicates an upgrade from precision polishing to what we may be defined as “ultra-precision” polishing and entered the era of ACS [16,17][16][17].
Polishing at ACS is defined as a series of methods to realize surface roughness in Ångström level, where the material removal mechanisms are established by quantum mechanics. Using current polishing procedures, roughness in Ångström level represents the highest level of surface finish achievable.
The chart of machining accuracy over time proposed by Taniguchi in 1983 [23][18] is now verified in practice [24][19]. Atomic and close-to-atomic scale manufacturing (ACSM) is the next generation of manufacturing technology and will be the leading trend in developing Manufacturing III [25][20]. Roughness in Ångström level is not only essential, but more importantly, achievable through polishing at ACS [26][21]. For instance, substrates mounted in ring laser gyroscopes are required to have a surface with a roughness of 0.5 nm in Ra [24][19]. For a hard X-ray system, 0.2 nm in RMS is required for mirrors [27,28][22][23]. In the field of ultraviolet lithography, 0.3 nm in RMS [29][24] and 0.15 nm in RMS [30,31][25][26] are needed by the lens. The roughness of a PC hard disc must be less than 0.05 nm in Ra to achieve a storage density larger than 500~1000 Gb/in2 [32][27].
The above examples indicate how the demands for polishing at ACS have become increasingly stringent [33][28], with the development of information technology [32][27], laser technology [34][29], optical industry [35[30][31],36], electronic power devices [37][32], and physical sciences [38,39][33][34].
2. Chemical Modification Polishing Approaches
2.1. Chemical Mechanical Polishing
Robert introduced CMP to polish semiconductor materials in 1965 [53][35]. The International Business Machines Corporation (IBM) began utilizing CMP technology in dynamic random access memory manufacturing in 1988 [54][36]. Since then, IBM [55][37] and other researchers have investigated and progressed CMP technology continuously.2.1.1. Removal Mechanism
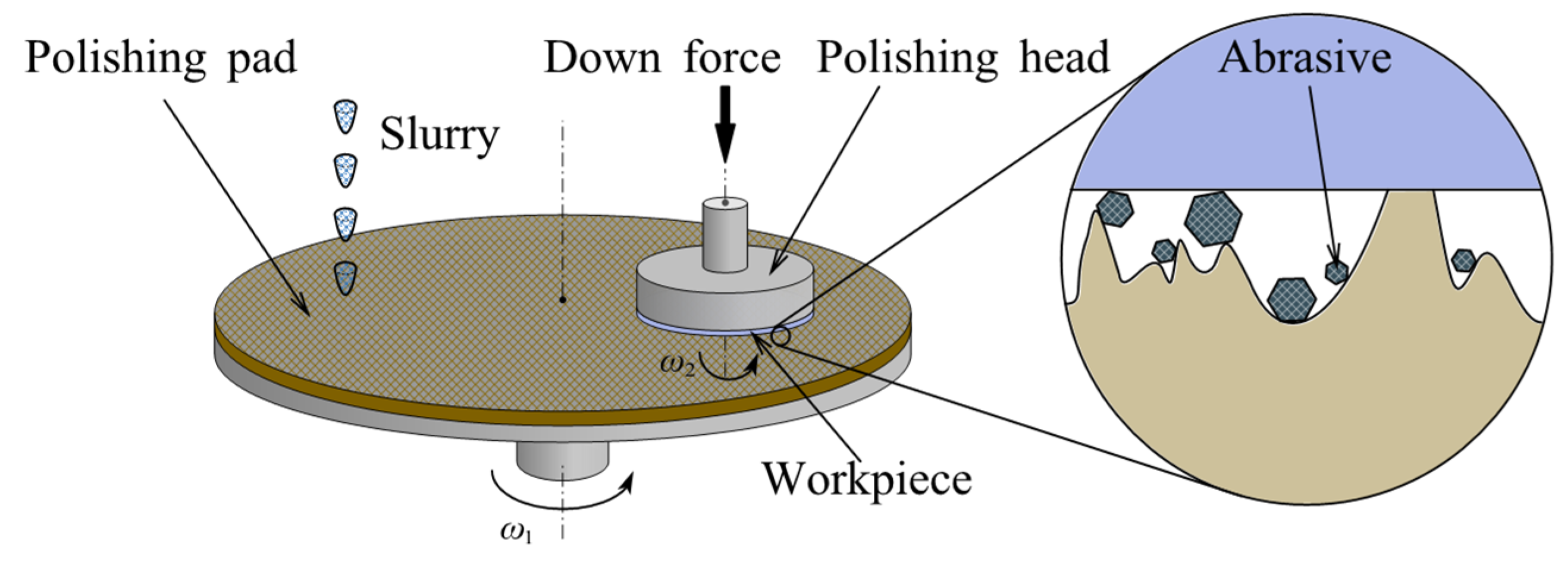
CMP is a technique for atomic-level removal. The chemical reaction of the polishing slurry and the mechanical interaction of the abrasive are combined to remove material [56,57][38][39]. The workpiece is fixed on the polishing workhead spindle and loaded downward against the polishing pad. Both the polishing head and pad are driven by servo motors with adjustable speed. The chemical action of the polishing slurry softens a thin layer on the workpiece surface, which is subsequently removed by the mechanical interaction of upcoming abrasives to obtain an ultra-smooth surface [58][40], as shown in Figure 41. The removal mechanism of CMP was studied from the following three aspects, including chemical reaction, mechanical interaction, and edge effect. (1) The chemical reaction modifies the surface material of the workpiece to facilitate subsequent mechanical removal. In the early 1990s, Cook [59][41] proposed the chemical bonding removal model. It describes how abrasive atoms/molecules interact with workpiece atoms/molecules to form chemical bonds. Based on the chemical bonding removal model, the chemical modification of Si was analyzed using molecular dynamics. For instance, when CeO2 was used as the abrasives, a Ce-O-Si bridging bond formed at the interface between workpiece and abrasive particle. This condition causes instability of the pyramidal structure of the material, thus breaking the original Si-O bond [60][42]. This instability of the bridging bonds on the workpiece surface provides the basis for removal by subsequent mechanical interaction. (2) Mechanical interaction removes the material directly after chemical modification. (3) The fluid floating and tool roll-off effect influences P in Preston equation. Although the erosion effect of the polishing fluid on the polished material is almost negligible [66][43], based on the Reynolds equation [67][44], fluid pressure affects the hydrodynamic lubrication properties of the slurry and the dynamic balance in CMP [68][45]. During the polishing process, the fluid floating effect caused by the hydrodynamics lifts the workpiece leading edge up. As a result, there is negative pressure at the edge of the workpiece [68][45]. Tool roll-off effect refers to the fact that as polishing pad progressively hangs over the edge of the workpiece, the pressure naturally diminishes due to the smaller and smaller contact area. This edge effect deteriorates the form accuracy of the workpiece. According to the removal mechanism in CMP, both mechanical and chemical action play an equally crucial role in achieving material removal. Maintaining the balance between these two actions is the key to obtaining sub-nanometer roughness.2.1.2. Chemical Reaction Factors
The recipe of the CMP slurry determines the process and rate of chemical reactions. Typically, the recipe includes abrasive particles, oxidizers, surfactants, and deionized (DI) water [69][46]. Material removal is mostly determined by the type of abrasive particles. The chemical reactions between the polishing slurry and the workpiece are determined by the surface charges. As a result, the material removal rate and roughness are determined by the acidity or alkalinity of the slurry [74][47]. For instance, the removal of Si was shown to be highest under alkaline conditions, as the OH termination increases with solution pH and strong polarization weakens Si–Si back bonds [75][48]. Acid slurries, instead, have been shown to reduce roughness while polishing tungsten alloy. For example, citric acid-based slurry balances mechanical and chemical processes and prevents grain boundary steps from developing, thus offering a superior surface finish [76][49]. Li et al. [74][47] used acid colloidal silica to obtain sub-nanometer roughness for YAG crystal, showing that the acidic slurry facilitates chemical reactions and the elimination of surface scratches or damages. From this exploration, one can conclude that to obtain sub-nanometer roughness, the optimal pH of polishing slurry needs to be explored depending on the workpiece material.2.1.3. Mechanical Interaction Factors
Mechanical interaction ultimately removes the material, also influencing the surface roughness. The mechanical abrasive process in CMP is divided into two-body abrasion and three-body abrasion. Under two-body abrasion, particles are trapped in the polishing pad, while the hard protuberances slide on the workpiece surfaces. Under the three-body abrasion model, abrasive particles are free to roll and slide [77][50]. Experiment [78][51] and simulation [79][52] showed that the material removal rate in three-body abrasion is one order of magnitude less than that for two-body abrasion. Pressure has a direct impact on roughness when polishing single-crystal diamond (SCD). If there is only mechanical interaction without chemical reaction to modify the top of the workpiece surface, this polishing is called mechanical polishing. Unlike mechanical polishing, which requires considerable pressure to achieve material removal [80][53], CMP can polish high-hardness materials with ACS precision at atmospheric pressure.2.1.4. Characteristics
By combining chemical and mechanical actions, CMP can produce ultra-smooth surfaces with greater precision than machining equipment, as shown in Table 1.Table 1.
The ultimate roughness achieved with CMP for different materials.
- (1)
-
While softening the workpiece surface, the polishing slurry also has a corrosive effect on the polishing pads, resulting in more frequent replacement of polishing pads and higher costs.
- (2)
-
During the polishing of metals, the workpiece is easily scratched by the abrasive grains due to the low hardness, making it difficult to achieve sub-nanometer roughness [91,92,93][62][63][64]. Trial and error are inevitable to find an appropriate slurry recipe when CMP is applied to new metal material.
- (3)
-
Low contact pressure is necessary to achieve low roughness, but unavoidably affects removal efficiency. As loose abrasive particles rarely slide on the workpiece surface under low contact pressure, they spend about 90% of their time rolling according to the three-body abrasion mode [77][50].
- (4)
-
The workpiece roughness under three-body abrasion is sensitive to the abrasive size. When the abrasive size is not uniform, the mechanical material removal is carried out by a small number of large abrasives, probably resulting in the formation of scratches, pits, and other damage.
References
- Zucuni, C.P.; Dapieve, K.S.; Rippe, M.P.; Pereira, G.K.R.; Bottino, M.C.; Valandro, L.F. Influence of finishing/polishing on the fatigue strength, surface topography, and roughness of an yttrium-stabilized tetragonal zirconia polycrystals subjected to grinding. J. Mech. Behav. Biomed. Mater. 2019, 93, 222–229.
- Ettl, P.; Schmidt, B.E.; Schenk, M.; Laszlo, I.; Haeusler, G. Roughness parameters and surface deformation measured by coherence radar. Int. Conf. Appl. Opt. Metrol. 1998, 3407, 133–140.
- Kriiska, A. Excavations of the Stone Age site at Vihasoo III. Arheol. Välitööd Eest. 1996, 7, 19–28.
- Helden, V.; Albert; Dupré, S.; Gent, R.V. The Origins of the Telescope; Amsterdam University Press: Amsterdam, The Netherlands, 2010.
- Masters, B.R. Ernst Abbe and the Foundation of Scientific Microscopes. Opt. Photonics News 2007, 18, 18.
- Carl Zeiss, Ernst Abbe and Otto Schott–A Winning Team. 2002. Available online: https://www.zeiss.com/vision-care/int/better-vision/understanding-vision/carl-zeiss-ernst-abbe-and-otto-schott-a-winning-team.html (accessed on 15 December 2022).
- Clay, W. Surface Finish. Proc. Inst. Automob. Eng. 1944, 38, 43–73.
- Pomes, C.; Slack, G.; Wise, M. Surface roughness of dental castings. J. Am. Dent. Assoc. 1950, 41, 545–556.
- Schlesinger, G. Surface finish and the function of part. Proc. Inst. Mech. Eng. 1944, 151, 153–178.
- Pimenov, S.M.; Kononenko, V.V.; Ralchenko, V.G.; Konov, V.I.; Gloor, S.; Lüthy, W.; Weber, H.P.; Khomich, A.V. Laser polishing of diamond plates. Appl. Phys. A: Mater. Sci. Process. 1999, 69, 81–88.
- Higashi, Y.; Meike, T.; Suzuki, J.; Mori, Y.; Yamauchi, K.; Endo, K.; Namba, H. New machining method for making precise and very smooth mirror surfaces made from Cu and Al alloys for synchrotron radiation optics. Rev. Sci. Instrum. 1989, 60, 2120–2123.
- Fähnle, O.W.; van Brug, H.H. Fluid jet polishing: Removal process analysis. Opt. Fabr. Test. 1999, 3739, 68–77.
- Basim, G.; Adler, J.; Mahajan, U.; Singh, R.; Moudgil, B. Effect of particle size of chemical mechanical polishing slurries for enhanced polishing with minimal defects. J. Electrochem. Soc. 2000, 147, 3523.
- Muratov, V.A.; Fischer, T.E. Tribochemical polishing. Annu. Rev. Mater. Sci. 2000, 30, 27–51.
- Lee, B.; Quinn, L.; Baine, P.; Mitchell, S.; Armstrong, B.; Gamble, H. Investigation of the role of chemical-mechanical polishing in improving the performance of polysilicon TFTs. MRS Online Proc. Libr. (OPL) 1999, 558, 257–262.
- Mathew, P.T.; Rodriguez, B.J.; Fang, F.Z. Atomic and Close-to-Atomic Scale Manufacturing: A Review on Atomic Layer Removal Methods Using Atomic Force Microscopy. Nanomanufacturing Metrol. 2020, 3, 167–186.
- Fang, F.Z. On Atomic and Close-to-atomic Scale Manufacturing—Development Trend of Manufacturing Technology. China Mech. Eng. 2020, 31, 1009–1021.
- Taniguchi, N. Current Status in, and Future Trends of, Ultraprecision Machining and Ultrafine Materials Processing. CIRP Ann. 1983, 32, 573–582.
- Zhang, Z.; Yan, J.; Kuriyagawa, T. Manufacturing technologies toward extreme precision. Int. J. Extrem. Manuf. 2019, 1, 022001.
- Fang, F.Z. Atomic and close-to-atomic scale manufacturing: Perspectives and measures. Int. J. Extrem. Manuf. 2020, 2, 030201.
- Fang, F.Z.; Zhang, X.; Gao, W.; Guo, Y.; Byrne, G.; Hansen, H.N. Nanomanufacturing—Perspective and applications. CIRP Ann. 2017, 66, 683–705.
- Assoufid, L.; Ohashi, H.; Asundi, A.K.; Yumoto, H.; Koyama, T.; Matsuyama, S.; Yamauchi, K.; Ohashi, H. Ultra-high-precision surface processing techniques for nanofocusing ellipsoidal mirrors in hard x-ray region. Adv. Metrol. X-Ray EUV Opt. V 2014, 9206, 27–37.
- Usuki, K.; Mazuray, L.; Kitayama, T.; Wartmann, R.; Wood, A.P.; Matsumura, H.; Kojima, T.; de la Fuente, M.C.; Tissot, J.-L.M.; Uchikoshi, J.; et al. Development of a nanoprofiler using the follow-up normal vector to the surface for next-generation ultraprecise mirrors. Optical Systems Design 2012 2012, 8550, 220–227.
- Kurashima, Y.; Miyachi, S.; Miyamoto, I.; Ando, M.; Numata, A. Evaluation of surface roughness of ULE® substrates machined by Ar+ ion beam. Microelectron. Eng. 2008, 85, 1193–1196.
- Kanaoka, M.; Liu, C.; Nomura, K.; Ando, M.; Takino, H.; Fukuda, Y.; Mori, Y.; Mimura, H.; Yamauchi, K. Processing efficiency of elastic emission machining for low-thermal-expansion material. Surf. Interface Anal. 2008, 40, 1002–1006.
- Hasan, R.M.M.; Luo, X. Promising Lithography Techniques for Next-Generation Logic Devices. Nanomanufacturing Metrol. 2018, 1, 67–81.
- Xu, J.; Luo, J.; Lu, X.; Zhang, C.; Pan, G. Progress in material removal mechanisms of surface polishing with ultra precision. Chin. Sci. Bull. 2004, 49, 1687–1693.
- Yin, S.H.; Wang, Y.Q.; Li, Y.P.; Gong, S. Experimental Study on Effects of Translational Movement on Surface Planarity in Magnetorheological Planarization Process. Adv. Mater. Res. 2016, 1136, 293–296.
- Gautam, G.D.; Pandey, A.K. Pulsed Nd:YAG laser beam drilling: A review. Opt. Laser Technol. 2018, 100, 183–215.
- Schuelke, T.; Grotjohn, T.A. Diamond polishing. Diam. Relat. Mater. 2013, 32, 17–26.
- Chkhalo, N.I.; Kaskov, I.A.; Malyshev, I.V.; Mikhaylenko, M.S.; Pestov, A.E.; Polkovnikov, V.N.; Salashchenko, N.N.; Toropov, M.N.; Zabrodin, I.G. High-performance facility and techniques for high-precision machining of optical components by ion beams. Precis. Eng. 2017, 48, 338–346.
- Kohn, E.; Adamschik, M.; Schmid, P.; Denisenko, A.; Aleksov, A.; Ebert, W. Prospects of diamond devices. J. Phys. D Appl. Phys. 2001, 34, 77–85.
- Yamauchi, K.; Mimura, H.; Inagaki, K.; Mori, Y. Figuring with subnanometer-level accuracy by numerically controlled elastic emission machining. Rev. Sci. Instrum. 2002, 73, 4028–4033.
- Khounsary, A.; Takei, Y.; Kume, T.; Motoyama, H.; Hiraguri, K.; Hashizume, H.; Mimura, H.; Goto, S.; Morawe, C. Development of a numerically controlled elastic emission machining system for fabricating mandrels of ellipsoidal focusing mirrors used in soft x-ray microscopy. Adv. X-Ray/EUV Opt. Compon. VIII 2013, 8848, 71–80.
- Walsh, R.J.; Arno, H.H. Process for Polishing Semi-conductor Materials. U.S. Pat. Trademark Off. 1965, 3, 273.
- Fury, M.A. The early days of CMP. Solid State Technol. 1997, 40, 81–84.
- Zantye, P.B.; Kumar, A.; Sikder, A.K. Chemical mechanical planarization for microelectronics applications. Mater. Sci. Eng. R Rep. 2004, 45, 89–220.
- Pan, B.; Kang, R.; Guo, J.; Fu, H.; Du, D.; Kong, J. Precision Fabrication of Thin Copper Substrate by Double-sided Lapping and Chemical Mechanical Polishing. J. Manuf. Process. 2019, 44, 47–54.
- Guo, J.; Shi, X.; Song, C.; Niu, L.; Cui, H.; Guo, X.; Tong, Z.; Yu, N.; Jin, Z.; Kang, R. Theoretical and experimental investigation of chemical mechanical polishing of W–Ni–Fe alloy. Int. J. Extrem. Manuf. 2021, 3, 025103.
- Geng, Z.; Zhou, P.; Meng, L.; Yan, Y.; Guo, D. Prediction of Surface Profile Evolution of Workpiece and Lapping Plate in Lapping Process. J. Manuf. Sci. Eng. 2022, 144, 081001.
- Cook, L.M. Chemical processes in glass polishing. J. Non-Cryst. Solids 1990, 120, 152–171.
- Onodera, T.; Takahashi, H.; Nomura, S. First-principles molecular dynamics investigation of ceria/silica sliding interface toward functional materials design for chemical mechanical polishing process. Appl. Surf. Sci. 2020, 530, 147259.
- Lin, J.F.; Chern, J.D.; Chang, Y.H.; Kuo, P.L.; Tsai, M.S. Analysis of the tribological mechanisms arising in the chemical mechanical polishing of copper-film wafers. J. Tribol. 2004, 126, 185–199.
- Reynolds, O., IV. On the theory of lubrication and its application to Mr. Beauchamp tower’s experiments, including an experimental determination of the viscosity of olive oil. Philos. Trans. R. Soc. Lond. 1886, 1, 157–234.
- Zhao, D.; Wang, T.; He, Y.; Lu, X. Experimental studies on interfacial fluid lubrication and wafer status during chemical mechanical polishing of 12-inch wafer. In Proceedings of the ICPT 2012-International Conference on Planarization/CMP Technology, Grenoble, France, 15–17 October 2012; pp. 1–6.
- Li, J.; Liu, Y.; Dai, Y.; Yue, D.; Lu, X.; Luo, J. Achievement of a near-perfect smooth silicon surface. Sci. China Technol. Sci. 2013, 56, 2847–2853.
- Li, J.; Zhu, Y.; Chen, C.T. Chemical Mechanical Polishing of Transparent Nd:YAG Ceramics. Key Eng. Mater. 2008, 375–376, 278–282.
- Katsuki, F.; Kamei, K.; Saguchi, A.; Takahashi, W.; Watanabe, J. AFM studies on the difference in wear behavior between Si and SiO2 in KOH solution. J. Electrochem. Soc. 2000, 147, 2328.
- Guo, J.; Song, C.; Niu, L.; Shi, X.; Jin, Z.; Guo, C.; Namba, Y. Suppression of grain boundary steps in chemical mechanical polishing of W-Ni-Fe alloy by a citric acid-based slurry. Manuf. Lett. 2020, 25, 40–43.
- Harsha, A.; Tewari, U. Two-body and three-body abrasive wear behaviour of polyaryletherketone composites. Polym. Test. 2003, 22, 403–418.
- Gahr, K.-H.Z. Wear by hard particles. Tribol. Int. 1998, 31, 587–596.
- Lin, Y.-Y.; Lo, S.-P. Finite element modeling for chemical mechanical polishing process under different back pressures. J. Mater. Process. Technol. 2003, 140, 646–652.
- Chen, Y.; Liangchi, Z. Polishing of Diamond Materials: Mechanisms, Modeling and Implementation; Springer: New York, NY, USA, 2013.
- Yamazaki, T.; Kurokawa, S.; Umezaki, Y.; Ohnishi, O.; Akagami, Y.; Yamaguchi, Y.; Kishii, S. Study on the Development of Resource-Saving High Performance Slurry-Polishing/CMP for glass substrates in a radical polishing environment, using manganese oxide slurry as an alternative for ceria slurry. Adv. Sci. Technol. 2010, 64, 65–70.
- Aida, H.; Doi, T.; Takeda, H.; Katakura, H.; Kim, S.-W.; Koyama, K.; Yamazaki, T.; Uneda, M. Ultraprecision CMP for sapphire, GaN, and SiC for advanced optoelectronics materials. Curr. Appl. Phys. 2012, 12, S41–S46.
- Thomas, E.L.; Mandal, S.; Brousseau, E.B.; Williams, O.A. Silica based polishing of and single crystal diamond. Sci. Technol. Adv. Mater. 2014, 15, 035013.
- Zhou, Y.; Pan, G.; Shi, X.; Gong, H.; Luo, G.; Gu, Z. Chemical mechanical planarization (CMP) of on-axis Si-face SiC wafer using catalyst nanoparticles in slurry. Surf. Coat. Technol. 2014, 251, 48–55.
- Shi, X.; Pan, G.; Zhou, Y.; Xu, L.; Zou, C.; Gong, H. A study of chemical products formed on sapphire (0001) during chemical–mechanical polishing. Surf. Coat. Technol. 2015, 270, 206–220.
- Shi, X.; Chen, G.; Xu, L.; Kang, C.; Luo, G.; Luo, H.; Zhou, Y.; Dargusch, M.S.; Pan, G. Achieving ultralow surface roughness and high material removal rate in fused silica via a novel acid SiO2 slurry and its chemical-mechanical polishing mechanism. Appl. Surf. Sci. 2020, 500, 144041.
- Zhang, Z.; Jin, Z.; Guo, J. The effect of the interface reaction mode on chemical mechanical polishing. CIRP J. Manuf. Sci. Technol. 2020, 31, 539–547.
- Wang, W.; Chen, Y.; Chen, A.; Ma, X. Composite particles with dendritic mesoporous-silica cores and nano-sized CeO2 shells and their application to abrasives in chemical mechanical polishing. Mater. Chem. Phys. 2020, 240, 122279.
- Xu, Q.; Chen, L.; Yang, F.; Cao, H. Influence of slurry components on copper CMP performance in alkaline slurry. Microelectron. Eng. 2017, 183, 1–11.
- Lei, H.; Jiang, L.; Chen, R. Preparation of copper-incorporated mesoporous alumina abrasive and its CMP behavior on hard disk substrate. Powder Technol. 2012, 219, 99–104.
- Liao, C.; Guo, D.; Wen, S.; Luo, J. Effects of chemical additives of CMP slurry on surface mechanical characteristics and material removal of copper. Tribol. Lett. 2012, 45, 309–317.
More