One of the major problems in food science is meeting the demand of the world’s growing population, despite environmental limitations such as climate change, water scarcity, land degradation, marine pollution, and desertification. Preventing food from going to waste and utilizing nutritive by-products as food rather than feed are easy and powerful strategies for overcoming this problem. Rice is an important staple food crop for more than half of the world’s population and substantial quantities of rice bran emerge as the main by-product of rice grain milling. Usually, rice bran is used as animal feed or discarded as waste. Although it is highly nutritious and comprises many bioactive compounds with considerable health benefits, the rapid deterioration of bran limits the exploitation of the full potential of rice bran. Hydrolytic rancidity is the main obstacle to using rice bran as food, and the enzyme inactivation process, which is termed stabilization, is the only way to prevent it.
- rice bran
- stabilization
- by-product
- utilization
1. Introduction
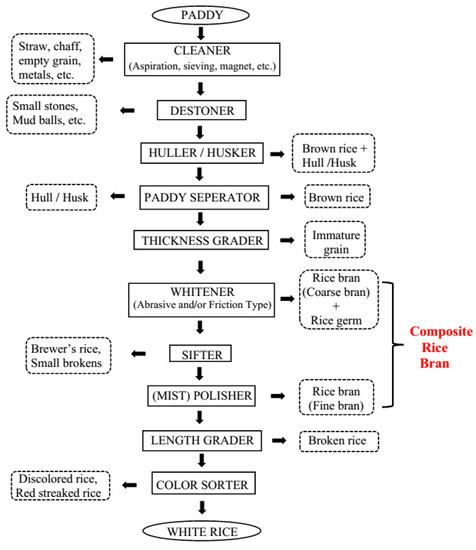
2. Rice Bran Stabilization Methods
2.1. Extrusion
2.2. Microwave Heating
2.3. Dry or Moist Heat Treatments
2.4. Infrared Heating
2.5. Ohmic Heating
2.6. Radio Frequency Heating
2.7. Irradiation
2.8. Other Stabilization Approaches
Pourali et al. (2009) employed subcritical water extraction as an environmentally friendly technique to stabilize and extract RB oil simultaneously [82]. Researchers also conducted conventional solid–liquid extraction (hexane–bran, hexane–water–bran, and ethanol–bran) for comparison. No increase was observed in the content of FFA in subcritical-water-extracted and ethanol-extracted (60 °C) RB oil, while FFA concentration increased from 5.0% to 5.6% and from 6.5% to 7.0% in hexane-extracted (25 °C) and hexane–water-extracted (25 °C) RB oils, respectively, after 12 weeks of storage [82]. Another interesting approach was performed by Raghavendra et al. (2017) [83]. It is known that polyphenols can bind proteins and enzymes and alter their structural properties and biological activities. Raghavendra et al. (2017) showed that the activity of isolated and purified RB lipase decreased in the presence of chlorogenic and prominently caffeic acids. Researchers found 56% loss of lipase activity at 60 µM caffeic acid concentration. The loss of enzymatic activity increased with increasing concentration of the noted ligands [83].
Gopinger et al. (2015) treated RB with a mixture of acetic and propionic acids (1:1, m/m) with the aim of stabilization. The organic acid mixture (2% based on bran weight) was applied via spraying and the bran samples were stored at +18 °C for 120 days. Although the authors did not analyze typical stabilization indicators such as lipase activity and FFA content, lower lipid acidity (titratable acidity) increase and less lipid oxidation product formation were reported in organic acid-treated RB [84]. Yu et al. (2020) compared various stabilization methods such as MW (700 W for 2, 4, 6 min), steam heating (for 20, 40, 60 min), dry heating (at 105 °C for 30, 60, 90 min), IR heating (at 105 °C for 30, 60, 90 min), autoclaving (at 121 °C for 20 min), extrusion (at 60, 65, 115, 120 °C subsequent heating, 400–500 rpm screw speed), enzyme treatment (pepsin and papain), low-temperature storage (at 4, −18, and −80 °C for 72 h), ultraviolet irradiation (at 254 nm for 6, 12, 18 h), and ultrasound (28 kHz and 300 W for 30, 60, 90 min) for acid value, lipase, and peroxidase activities [85]. The authors reported that autoclaving is the most effective method for lipase inactivation at the noted conditions. Significant decreases in acid values were found after MW, autoclaving, steam heating, low-temperature storage, IR heating, and extrusion treatments (p < 0.05). However, non-thermal methods were not effective in terms of lipase inactivation. Residual lipase activities of RB treated with UV radiation even for 18 h and RB stored at extreme low temperature (−80 °C for 72 h) were 57% and 58%, respectively. Moreover, ultrasound markedly increased the peroxide value of RB oil [85].
Parboiling is another practice employed for RB stabilization. Although the term “parboiling” does not define a specific condition, it generally refers to soaking the paddy in water (at varying temperatures) followed by a short steaming procedure and drying (solar drying in most cases). In general, the bran obtained from milling of parboiled paddy is used for extraction of RB oil [64].
References
- Kahlon, T.S. Rice bran: Production, composition, functionality and food applications, physiological benefits. In Fiber Ingredients: Food Applications and Health Benefits; Cho, S., Samuel, P., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 305–321.
- Basri, M.S.M.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Optimization of rice husk ash based geopolymers coating composite for enhancement in flexural properties and microstructure using response surface methodology. Coatings 2020, 10, 165.
- Rice Knowledge Bank, IRRIa. Available online: http://www.knowledgebank.irri.org/step-by-step-production/postharvest/rice-by-products/rice-husk (accessed on 16 February 2023).
- Mir, S.A.; Shah, M.A.; Bosco, S.J.D.; Sunooj, K.V.; Farooq, S. A review on nutritional properties, shelf life, health aspects, and consumption of brown rice in comparison with white rice. Cereal Chem. 2020, 97, 895–903.
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z.G. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096.
- Selvam, S.; Masilamani, P.; Umashankar, P.T.; Albert, V.A. Opportunities and challenges in marketing of brown rice. In Brown Rice; Monickavasagan, A., Santhakumar, C., Venkatachalapathy, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 296–305.
- Rice Knowledge Bank, IRRIb. Available online: http://www.knowledgebank.irri.org/step-by-step-production/postharvest/milling/milling-systems?tmpl=component&print=1 (accessed on 17 February 2023).
- Saunders, R.M. Rice bran: Composition and potential food uses. Food Rev. Int. 1985, 1, 465–495.
- Malekian, F.; Rao, R.M.; Prinyawiwatkul, W.; Marshall, W.E.; Windhauser, M.; Ahmedna, M. Lipase and lipoxygenase activity, functionality, and nutrient losses in rice bran during storage. Bull. La. Agric. Exp. Stn. 2000, 870, 1–68.
- Bhardwaj, K.; Raju, A.; Rajasekharan, R. Identification, purification, and characterization of a thermally stable lipase from rice bran. A new member of the (phospho) lipase family. Plant Physiol. 2001, 127, 1728–1738.
- Food and Agriculture Organization of the United Nations, FAOSTAT. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 February 2023).
- Espinales, C.; Cuesta, A.; Tapia, J.; Palacios-Ponce, S.; Penas, E.; Martinez-Villaluenga, C.; Espinoza, A.; Caceres, P.J. The effect of stabilized rice bran addition on physicochemical, sensory, and techno-functional properties of bread. Foods 2022, 11, 3328.
- Doan, N.T.T.; Lai, Q.D.; Vo, H.V.; Nguyen, H.D. Influence of adding rice bran on physio-chemical and sensory properties of bread. J. Food Meas. Charact. 2021, 15, 5369–5378.
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.B. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30.
- Tuncel, N.B.; Yılmaz, N.; Kocabıyık, H.; Uygur, A. The effect of infrared stabilized rice bran substitution on physicochemical and sensory properties of pan breads: Part I. J. Cereal Sci. 2014, 59, 155–161.
- Tuncel, N.B.; Yılmaz, N.; Kocabıyık, H.; Uygur, A. The effect of infrared stabilized rice bran substitution on B vitamins, minerals and phytic acid content of pan breads: Part II. J. Cereal Sci. 2014, 59, 162–166.
- Yılmaz Tuncel, N.; Kaya, E.; Karaman, M. Rice bran substituted Turkish noodles (Erişte): Textural, sensorial, and nutritional properties. Cereal Chem. 2017, 94, 903–908.
- Yılmaz, N.; Tuncel, N.B.; Kocabıyık, H. The effect of infrared stabilized rice bran substitution on nutritional, sensory, and textural properties of cracker. Eur. Food Res. Technol. 2014, 239, 259–265.
- Ayoub, W.S.; Zahoor, I.; Dar, A.H.; Anjum, N.; Pandiselvam, R.; Farooq, S.; Rusu, A.V.; Rocha, J.M.; Trif, M.; Jeevarathinam, G. Effect of incorporation of wheat bran, rice bran and banana peel powder on the mesostructure and physicochemical characteristics of biscuits. Front. Nutr. 2022, 9, 1016717.
- Akter, D.; Begum, R.; Rahman, M.N.; Talukder, N.; Alam, M.J. Optimization of extraction process parameter for rice bran protein concentrate and its utilization in high protein biscuit formulation. Curr. Res. Nutr. Food Sci. 2020, 8, 596–608.
- Albayrak, B.B.; Tuncel, N.B.; Yılmaz Tuncel, N.; Masatcioglu, M.T. Extrusion cooking of immature rice grain: Under-utilized by-product of rice milling process. J. Food Sci. Technol. 2020, 57, 2905–2915.
- Prakash, J. Rice bran proteins: Properties and food uses. Crit. Rev. Food Sci. Nutr. 1996, 36, 537–552.
- Echeverria, L.; da Silva, C.; Danesi, E.D.G.; Porciuncula, B.D.A.; Barros, B.C.B. Characterization of okara and rice bran and their application as fat substitutes in chicken nugget formulations. LWT—Food Sci. Technol. 2022, 161, 113383.
- Jiang, R.S.; Xiao, Z.G.; Huo, J.J.; Wang, H.G.; Li, H.; Su, S.; Duan, Y.M.; Gao, Y.Z. Effects of rice bran content on plant-based simulated meat: From the aspects of apparent properties and structural characteristics. Food Chem. 2022, 380, 131842.
- Chakraborty, M.; Budhwar, S.; Kumar, S. Potential of milling byproducts for the formulation of health drink and detox tea-substitute. J. Food Meas. Charact. 2022, 16, 3153–3165.
- Tran, K.N.; Gidley, M.J.; Fitzgerald, M. Opportunities and challenges in processing of by-product of rice milling protein as a food ingredient. Cereal Chem. 2017, 94, 369–376.
- Wang, N.; Cui, X.; Duan, Y.; Yang, S.; Wang, P.; Saleh, A.S.M.; Xiao, Z. Potential health benefits and food applications of rice bran protein: Research advances and challenges. Food Rev. Int. 2021, in press.
- Tu, Y.; Zhang, X.X.; Wang, L. Effect of salt treatment on the stabilization of Pickering emulsions prepared with rice bran protein. Food Res. Int. 2023, 166, 112537.
- Zheng, L.; Regenstein, J.M.; Wang, Z.J.; Zhang, H.J.; Zhou, L.Y. Reconstituted rice protein: The raw materials, techniques and challenges. Trends Food Sci. Technol. 2023, 133, 267–276.
- Magnaye, M.J.F.A.; Mopera, L.E.; Flores, F.P. Effect of rice bran protein concentrate as wall material adjunct on selected physicochemical and release properties of microencapsulated beta-carotene. Food Sci. Technol. Int. 2022, 28, 653–662.
- Bonifacino, C.; Palazolo, G.G.; Panizzolo, L.A.; Abirached, C. Study of emulsifying properties of soluble proteins obtained from defatted rice bran concentrate. J. Am. Oil Chem. Soc. 2021, 98, 851–860.
- Pang, M.; Kang, S.M.; Liu, L.; Ma, T.F.; Zheng, Z.; Cao, L.L. Physicochemical properties and cookie-making performance as fat replacer of wax-based rice bran oil oleogels. Gels 2023, 9, 13.
- Wang, Z.H.; Li, S.Z.; Zhang, M.; Yang, H.Y.; Li, G.; Ren, X.; Liang, S. Optimization of oil extraction from rice bran with mixed solvent using response surface methodology. Foods 2022, 11, 3849.
- Chen, H.S.; He, S.D.; Sun, H.J.; Li, Q.Y.; Gao, K.; Miao, X.Y.; Xiang, J.; Wu, X.J.; Gao, L.M.; Zhang, Y. A comparative study on extraction and physicochemical properties of soluble dietary fiber from glutinous rice bran using different methods. Separations 2023, 10, 90.
- Ismail, N.A.; Zhao, J. Effects of ultrasound and steam explosion treatments on the physicochemical properties of rice bran fibre. Pertenika J. Trop. Agric. Sci. 2022, 45, 893–918.
- Wang, L.; Duan, W.; Zhou, S.; Qian, H.F.; Zhang, H.; Qi, X.G. Effect of rice bran fibre on the quality of rice pasta. Int. J. Food Sci. Technol. 2018, 53, 81–87.
- Gu, X.Y.; Du, L.Y.; Meng, Z. Comparative study of natural wax-based W/O emulsion gels: Microstructure and macroscopic properties. Food Res. Int. 2023, 165, 112509.
- Dent, T.; Hallinan, R.; Chitchumroonchokchai, C.; Maleky, F. Rice bran wax structured oleogels and in vitro bioaccessibility of curcumin. J. Am. Oil Chem. Soc. 2022, 99, 299–311.
- Ghosh, S.; Bollinedi, H.; Krishnan, S.G.; Kundu, A.; Singh, A.; Bhowmick, P.K.; Singh, A.; Nagarajan, M.; Vinod, K.K.; Ellur, R.K.; et al. From farm to plate: Spatio-temporal characterization revealed compositional changes and reduced retention of gamma-oryzanol upon processing in rice. Front. Nutr. 2022, 9, 1040362.
- Zaky, A.A.; Chen, Z.; Qin, M.; Wang, M.Z.; Jia, Y.M. Assessment of antioxidant activity, amino acids, phenolic acids and functional attributes in defatted rice bran and rice bran protein concentrate. Prog. Nutr. 2020, 22, e2020069.
- Cosmetic Ingredient Review Expert Panel. Amended final report on the safety assessment of Oryza Sativa (rice) bran oil, Oryza Sativa (rice) germ oil, rice bran acid, Oryza Sativa (rice) bran wax, hydrogenated rice bran wax, Oryza Sativa (rice) bran extract, Oryza Sativa (rice) extract, Oryza Sativa (rice) germ powder, Oryza Sativa (rice) starch, Oryza Sativa (rice) bran, hydrolyzed rice bran extract, hydrolyzed rice bran protein, hydrolyzed rice extract, and hydrolyzed rice protein. Int. J. Toxicol. 2006, 2, 91–120.
- Tao, J.; Rao, R.; Liuzzo, J. Microwave heating for rice bran stabilization. J. Microw. Power Electromagn. Energy 1993, 28, 156–164.
- Yılmaz Tuncel, N.; Yılmaz Korkmaz, F. Comparison of lipid degradation in raw and infrared stabilized bran and rice bran oil: Matrix effect. J. Food Meas. Charact. 2021, 15, 1057–1067.
- Ju, Y.H.; Vali, S.R. Rice bran oil as a potential resource for biodiesel: A review. J. Sci. Ind. Res. 2005, 64, 866–882.
- Saji, N.; Schwarz, L.J.; Santhakumar, A.B.; Blanchard, C.L. Stabilization treatment of rice bran alters phenolic content and antioxidant activity. Cereal Chem. 2020, 97, 281–292.
- Pokkanta, P.; Yuenyong, J.; Mahatheeranont, S.; Jiamyangyuen, S.; Sookwong, P. Microwave treatment of rice bran and its effect on phytochemical content and antioxidant activity. Sci. Rep. 2022, 12, 7708.
- Rashid, M.T.; Liu, K.; Hen, S.; Jatoi, M.A.; Sarpong, F. Nutritional composition and volatile compounds stability in dry-heat and extruded stabilised rice bran during storage. Int. J. Food Sci. Technol. 2023, in press.
- Chen, Y.X.; Ma, Y.X.; Dong, L.H.; Jia, X.C.; Liu, L.; Huang, F.; Chi, J.W.; Xiao, J.; Zhang, M.W.; Zhang, R.F. Extrusion and fungal fermentation change the profile and antioxidant activity of free and bound phenolics in rice bran together with the phenolic bioaccessibility. LWT—Food Sci. Technol. 2019, 115, 108461.
- Randall, J.M.; Sayre, R.N.; Schultz, W.G.; Fong, R.Y.; Mossman, A.P.; Tribelhorn, R.E.; Saunders, R.M. Rice bran stabilization by extrusion cooking for extraction of edible oil. J. Food Sci. 1985, 50, 361–364.
- Kim, C.J.; Byun, S.M.; Cheigh, H.S.; Kwon, T.W. Optimization of extrusion rice bran stabilization process. J. Food Sci. 1987, 52, 1355.
- Fuh, W.S.; Chiang, B.H. Dephytinisation of rice bran and manufacturing a new food ingredient. J. Sci. Food Agric. 2001, 81, 1419–1425.
- Shin, T.S.; Godber, J.S.; Martin, D.E.; Wells, J.H. Hydrolytic stability and changes in E vitamers and oryzanol of extruded rice bran during storage. J. Food Sci. 1997, 62, 704–728.
- Sharma, H.R.; Chauhan, G.S.; Agrawal, K. Physico-chemical characteristics of rice bran processed by dry heating and extrusion cooking. Int. J. Food Prop. 2004, 7, 603–614.
- Escamillo-Castillo, B.; Varela-Montellano, R.; Sanchez-Tovar, S.A.; Solis-Fuentes, J.A.; Duran-de-Bazua, C. Extrusion deactivation of rice bran enzymes by pH modification. Eur. J. Lipid Sci. Technol. 2005, 107, 871–876.
- Rafe, A.; Sadeghian, A. Stabilization of Tarom and Domesiah cultivars rice bran: Physicochemical, functional and nutritional properties. J. Cereal Sci. 2017, 74, 64–71.
- Ramezanzadeh, F.M.; Rao, R.M.; Windhause, M.; Prinyawiwatkul, W.; Tulley, R.; Marshall, W.E. Prevention of hydrolytic rancidity in rice bran during storage. J. Agric. Food Chem. 1999, 47, 3050–3052.
- Ramezanzadeh, F.M.; Rao, R.M.; Windhause, M.; Prinyawiwatkul, W.; Marshall, W.E.; Windhauser, M. Effects of microwave heat, packaging, and storage temperature on fatty acid and proximate compositions in rice bran. J. Agric. Food Chem. 2000, 48, 464–467.
- Patil, S.S.; Kar, A.; Mohapatra, D. Stabilization of rice bran using microwave: Process optimization and storage studies. Food Bioprod. Process. 2016, 99, 204–211.
- Li, B.; Zhao, L.; Xu, B.; Deng, B.; Liu, Y.; Dong, Y. Rice bran real-time stabilization technology with flowing microwave radiation: Its impact on rancidity and some bioactive compounds. Qual. Assur. Saf. Crops Foods 2018, 10, 25–34.
- Lavanya, M.N.; Saikiran, K.C.H.; Venkatachalapathy, N. Stabilization of rice bran milling fractions using microwave heating and its effect on storage. J. Food Sci. Technol. 2019, 56, 889–895.
- Abdul-Hamid, A.; Sulaiman, R.R.R.; Osman, A.; Saari, N. Preliminary study of the chemical composition of rice milling fractions stabilized by microwave heating. J. Food Compos. Anal. 2007, 20, 627–637.
- Ertürk, B.; Meral, R. The impact of stabilization on functional, molecular and thermal properties of rice bran. J. Cereal Sci. 2019, 88, 71–78.
- Thanonkaew, A.; Wongyai, S.; McClements, D.J.; Decker, E.A. Effect of stabilization of rice bran by domestic heating on mechanical extraction yield, quality, and antioxidant properties of cold-pressed rice bran oil (Oryza saltiva L.). LWT—Food Sci. Tech. 2012, 48, 231–236.
- Amarasinghe, B.M.W.P.K.; Kumarasiri, N.C.; Gongodavilage, N.G. Effect of method of stabilization on aqueous extraction of rice bran oil. Food Bioprod. Process. 2009, 87, 108–114.
- Orthoefer, F.T.; Eastman, J. Rice bran and oil. In Rice: Chemistry and Technology; Champagne, E.T., Ed.; Agricultural Research Service, Southern Regional Research Center, U.S. Department of Agriculture: New Orleans, LA, USA, 2004; Chapter 19; pp. 569–593.
- Brunschwiler, C.; Heine, D.; Kappeler, S.; Conde-Petit, B.; Nyström, L. Direct measurement of rice bran lipase activity for inactivation kinetics and storage stability prediction. J. Cereal Sci. 2013, 58, 272–277.
- Li, Y.; Gao, Y.; Wang, Y.; Fan, M.; Wang, L.; Qian, H. Analysis of the aroma volatile compounds in different stabilized rice bran during storage. Food Chem. 2023, 405, 134753.
- Yılmaz, N.; Tuncel, N.B.; Kocabıyık, H. Infrared stabilization of rice bran and its effects on γ-oryzanol content, tocopherols and fatty acid composition. J. Sci. Food Agric. 2014, 94, 1568–1576.
- Irakli, M.; Kleisiaris, F.; Mygdalia, A.; Katsantonis, D. Stabilization of rice bran and its effect on bioactive compounds content, antioxidant activity and storage stability during infrared radiation heating. J. Cereal Sci. 2018, 80, 135–142.
- Yan, W.; Liu, Q.; Wang, Y.; Tao, T.; Liu, B.; Liu, J.; Ding, C. Inhibition of lipid and aroma deterioration in rice bran by infrared heating. Food Bioprocess. Technol. 2020, 13, 1677–1687.
- Wang, T.; Khir, R.; Pan, Z.; Yuan, Q. Simultaneous rough rice drying and rice bran stabilization using infrared radiation heating. LWT—Food Sci. Technol. 2017, 78, 281–288.
- Atungulu, G.G.; Pan, Z. Infrared radiative properties of food materials. In Infrared Heating for Food and Agricultural Processing; Pan, Z., Atungulu, G.G., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 29–35.
- Kubo, M.T.K.; Siguemoto, E.S.; Funcia, E.S.; Augusto, P.E.D.; Curet, S.; Boillereaux, L.; Sastry, S.K.; Gut, J.A.W. Non-thermal effects of microwave and ohmic processing on microbial and enzyme inactivation: A critical review. Curr. Opin. Food Sci. 2020, 35, 36–48.
- Lakkakula, N.R.; Lima, M.; Walker, T. Rice bran stabilization and rice bran oil extraction using ohmic heating. Biosour. Technol. 2004, 92, 157–161.
- Loypimai, P.; Moonggarm, A.; Chottanom, P. Effects of ohmic heating on lipase activity, bioactive compounds and antioxidant activity of rice bran. Aust. J. Basic Appl. Sci. 2009, 3, 3642–3652.
- Dhingra, D.; Chopra, S.; Rai, D.R. Stabilization of raw rice bran using ohmic heating. Agric. Res. 2012, 1, 392–398.
- Ling, B.; Lyng, J.G.; Wang, S. Effects of hot air-assisted radio frequency heating on enzyme inactivation, lipid stability and product quality of rice bran. LWT—Food Sci. Technol. 2018, 91, 453–459.
- Chen, Y.H.; Yen, Y.F.; Chen, S.D. Effects of radio frequency heating on the stability and antioxidant properties of rice bran. Foods 2021, 10, 810.
- Liao, M.; Damayanti, W.; Xu, Y.; Zhao, Y.; Xu, X.; Zheng, Y.; Jiao, S. Hot air-assisted radio frequency heating for stabilization of rice bran: Enzyme activity, phenolic content, antioxidant activity and microstructure. LWT-Food Sci. Technol. 2020, 131, 109754.
- Shin, T.S.; Godber, J.S. Changes of endogenous antioxidants and fatty acid composition in irradiated rice bran during storage. J. Agric. Food Chem. 1996, 44, 567–573.
- Masamran, S.; Chookaew, S.; Tepsongkroh, B.; Supawong, S. Impact of gamma irradiation pre-treatment before subcritical water extraction on recovery yields and antioxidant properties of rice bran extract. Radiat. Phys. Chem. 2023, 207, 110834.
- Pourali, O.; Asghari, F.S.; Yoshida, H. Simultaneous rice bran oil stabilization and extraction using sub-critical water medium. J. Food Eng. 2009, 95, 510–516.
- Raghavendra, M.P.; Kumar, P.R.; Prakash, V. Mechanism of inhibition of rice bran lipase by polyphenols: A case study with chlorogenic acid and caffeic acid. J. Food Sci. 2007, 72, 412–419.
- Gopinger, E.; Ziegler, V.; Catalan, A.A.S.; Krabbe, E.L.; Elias, M.C.; Xavier, E.G. Whole rice bran stabilization using a short chain organic acid mixture. J. Stored Prod. Res. 2015, 61, 108–113.
- Yu, C.W.; Hu, Q.R.; Wang, H.W.; Deng, Z.Y. Comparison of 11 rice bran stabilization methods by analyzing lipase activities. J. Food Process. Preserv. 2020, 44, e14370.
