Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 4 by Camila Xu.
Hydrophobic thin films have attracted significant attention in both basic research and practical applications due to their unique properties. These thin films have undergone extensive study, and numerous efforts have been made to broaden their application fields to include areas such as oil hydrophobicity, hydrophobic anti-icing, and hydrophobic anti-corrosion. Additionally, fresh studies that explore approximate theories and fabrication techniques for hydrophobic thin films have emerged.
- hydrophobic
- wettability
- magnetron sputtering
- surfaces
1. Introduction
Hydrophobic thin films have attracted significant attention in both basic research and practical applications due to their unique properties [1][2][3][4][5][6][7][8][9][10]. Over the past few decades, these thin films have undergone extensive study, and numerous efforts have been made to broaden their application fields to include areas such as oil hydrophobicity [11][12], hydrophobic anti-icing [13], and hydrophobic anti-corrosion [14][15]. Additionally, fresh studies that explore approximate theories and fabrication techniques for hydrophobic thin films have emerged [16][17][18][19].
Hydrophobic surfaces have great potential in a number of industries and biomedical fields, among others. The protection of electrical wires and antennas from snowfall, autos with self-cleaning windows, ships with anti-corrosion coatings, metal refining, building glasses with dust-free coatings, the separation of oil and water, and textiles resistant to stains are a few examples that come to mind.
Building a micro–nano rough structure on hydrophobic substrates or chemically altering a hierarchically structured surface with a low-surface-energy material are the two main methods for creating hydrophobic thin films. Many preparation methods have been reported in the literature, including phase separation [20], plasma methods [21][22], chemical vapor deposition (CVD) [23][24], physical vapor deposition (PVD) [25], sol-gel processing [26][27], and others. However, these techniques differ in terms of their efficiency, cost, simplicity of use, and requirements for specialized reagents. Some techniques are also restricted to basic laboratory experiments, and much work still needs to be performed to prepare hydrophobic films on a commercial scale. Therefore, researchers are working to make these films easier to prepare, cheaper, more durable, and more functional.
PVD is a frequently used technique for the deposition of thin films on a substrate. PVD involves the transfer of material from a source, typically in the form of a solid or liquid, to a substrate under vacuum conditions. There are several methods of PVD deposition, including sputtering [28][29], evaporation [30][31], and pulsed laser deposition (PLD).
Magnetron sputtering is a widely used PVD method for producing hydrophobic thin films. While the creation of small-area films using magnetron sputtering has been well established, the fabrication of large-area films poses unique challenges. Achieving uniformity and precise control of deposition parameters over a large area is inherently more difficult due to factors such as edge effects, variations in gas flow, and substrate curvature. In the context of magnetron sputtering, the feasibility of preparing large-area films arises from several distinctive features of the technique [32][33][34][35]. Moreover, the precise control of deposition parameters, such as gas pressure, target-to-substrate distance, and power density, plays a crucial role in achieving uniformity and high-quality films over large areas. So, this method stands out for its feasibility of preparing large-area films and is widely used in the industry.
Some other exceptional qualities, such as capacity for mass production, environmental friendliness, low cost, and powerful adhesion between film and substrate, also draw much attention. A high voltage is applied across the target material, creating a high-energy plasma that causes atoms or ions to be ejected from the surface of the target. To achieve uniform film deposition, these particles are then deposited onto the rotating substrate in a high-vacuum deposition chamber. However, a thorough analysis of magnetron-sputtered hydrophobic thin films is still lacking.
Hydrophobic films may be affected by environmental factors (such as ultraviolet rays, high temperatures, humidity, etc.) and physical or chemical effects (such as friction, corrosion, etc.) during long-term use, resulting in a decrease in or failure of their hydrophobic ability. Hydrophobic films have a number of significant characteristics that make them suitable for a variety of real-world uses. First, hydrophobic durability describes the thin film’s capacity to retain its hydrophobic qualities over time, even after exposure to environmental factors. In many applications, particularly those where water or moisture can cause damage or impair performance, hydrophobic durability is crucial. For instance, hydrophobic thin films can extend the lifespan and increase the reliability of sensitive electronics components by shielding them from water damage. Second, the ability to withstand exposure to harsh environments and to a variety of chemicals, including acids, bases, and solvents, is the second requirement for chemical stability in hydrophobic films. Additionally, they ought to be strong enough to withstand everyday wear and tear, as well as exposure to harsh temperatures and UV rays. Third, the hydrophobic film needs to be transparent in some applications, such as optics or electronics. Finally, biocompatibility, abrasion resistance, etc., are also significant in some other applications.
2. Principle of Hydrophobicity
2.1. Nature Inspiration
Numerous distinctive natural surfaces, such as lotus leaves, butterfly wings, cicada wings, and rose petals, offer fresh concepts for human designers of hydrophobic thin films. The lotus leaf primarily displays excellent superhydrophobicity; when it rains, water beads form on the leaves. The water beads roll away from the leaves as long as they are slightly tilted. The “Lotus Effect,” also known as the self-cleaning effect, was first discovered in the 1970s by a group of German botanical classification scientists led by W. Bartroot. ThereFigure 1 are shows some natural hydrophobic surfaces for a better understanding. Two popular techniques for producing the “Lotus Effect” on surfaces are nanoimprinting and anodic aluminum oxide techniques. With the help of pressure and heat, a mold having nano-scaled patterns is replicated onto a polymer substrate using the nanoimprinting technique. With this method, a surface topography that resembles the roughness of a lotus leaf is produced. The fabrication of the nano mold can be performed in a number of ways, but the most popular way to create a superhydrophobic surface is to copy a natural leaf or use deep reactive-ion etching on a silicon or silicon oxide substrate [36]. On the other hand, the anodic aluminum oxidation technique is a method that uses anodized templates as molds to grow a highly ordered nanotube structure on an aluminum surface. The surface of the resulting structure is hydrophobic due to its surface roughness [37]. The “Lotus Effect” is a remarkable phenomenon that displays superhydrophobicity. This effect has inspired researchers to develop hydrophobic thin films that exhibit self-cleaning properties [38][39][40][41][42].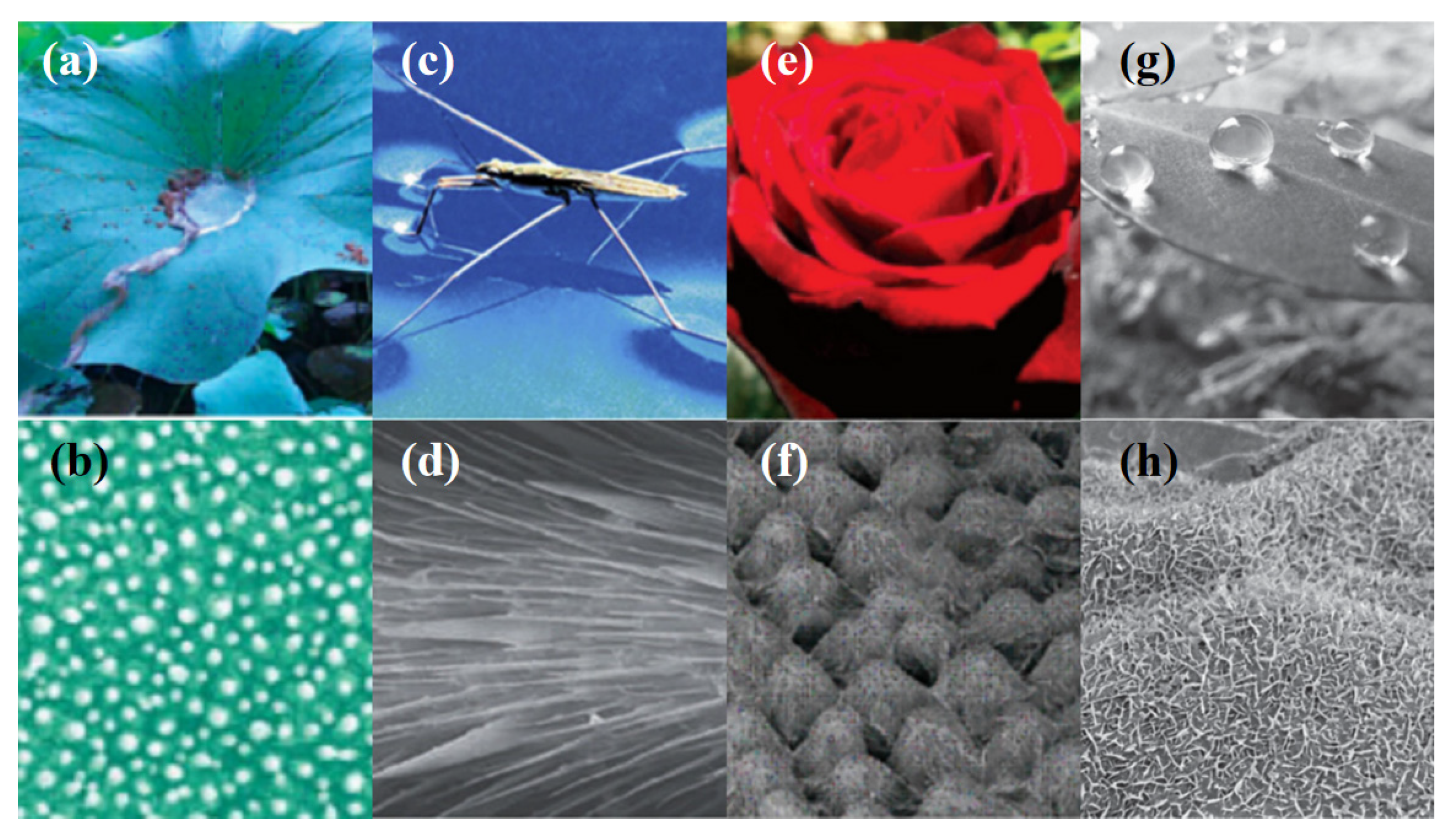
Figure 1. Natural hydrophobic surfaces. (a) Water beads formed on a lotus leaf shows the “Lotus Effect”. Reprinted with permission from ref. [7]. Copyright 2015 American Chemical Society. (b) Scanning electron microscope (SEM) image of the lower surface of the lotus leaf. Reprinted with permission from ref. [38]. Copyright 2002 Wiley. (c) Water strider legs show superhydrophobicity. Reproduced with permission from ref. [39]. Copyright 2006 Wiley. (d) SEM images of a water strider leg showing numerous oriented spindly microsetae. Reprinted with permission from ref. [40]. Copyright 2004 Nature Pub-lishing Group. (e,f) SEM images of the surface of a red rose petal, showing a periodic array of micropapillae and nanofolds on each papillae top. Reproduced with permission from ref. [41]. Copyright 2008 American Chemical Society. (g,h) Water droplets with spherical shape pinned on the irregular surface of peanut leaves and SEM image shows the top of nanoslices are covered with nanostructured papillae. Reproduced with per-mission from ref. [42]. Copyright 2013 Wiley.
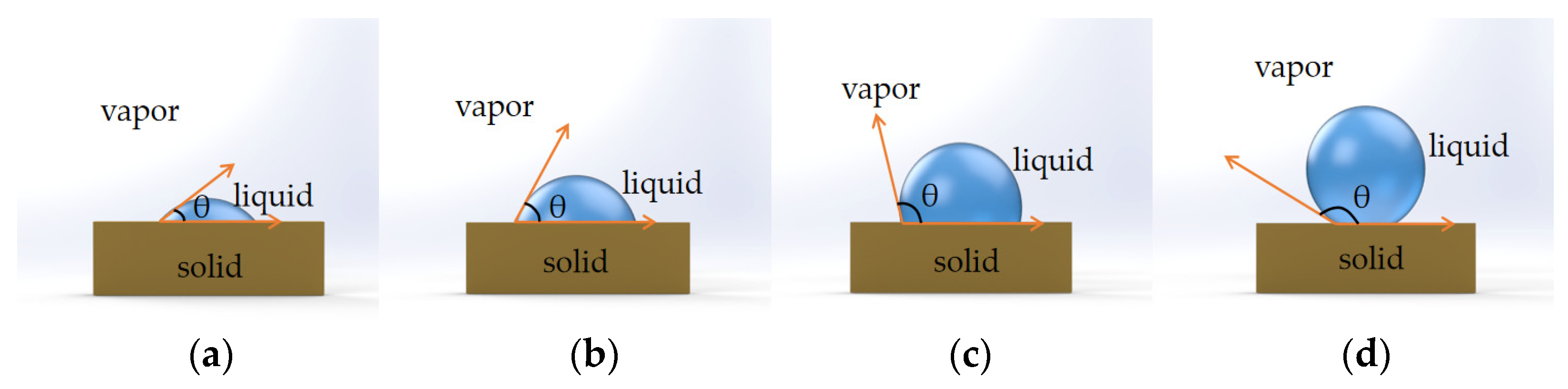
Figure 12. Wetting behavior of a liquid droplet on solid substrates with different contact angles. (a) superhydrophilic: θ < 5° in 0.5 s. (b) Hydrophilic: θ < 90°. (c) Hydrophobic: θ = 90°~150°. (d) Superhydrophobic: θ = 150~180°.
2.2. Young Equation
In 1805, Young was the first to propose the concept of contact angle to describe surface wettability, and he was the pioneer of research on wetting [44]. A liquid rests in a droplet on an ideal flat surface, as shown in Figure 23a. The surface free energy of a solid surface is expressed by the Young equation: where γsv, γsl, and γlv stand for the interfacial energy values for solid–vapor, solid-liquid, and liquid–vapor, respectively, and θY is the contact angle in the Young model. The Young angle is the result of the thermodynamic equilibrium of the surface free energy at the solid–liquid–vapor interface.2.3. Wenzel Model
However, there are not many perfect flat surfaces in the natural world. Wenzel developed the following equation in 1936 to establish a relationship between the macroscopic roughness of a solid surface and the contact angle, explaining how surface roughness increases hydrophobicity [45]: where θW is the apparent contact angle in the Wenzel model, r is the factor of surface roughness, and θY is the contact angle in the Young model. According to Wenzel’s theory, when a liquid comes into contact with a rough surface, it completely fills the voids and grooves of the surface, as seen in Figure 23b; as a result, the static contact angle is decreased, and the sliding angle is increased. Wenzel’s theory states that as roughness increases, hydrophilic surfaces become more wettable, while hydrophobic surfaces become less wettable.2.4. Cassie–Baxter Model
Cassie and Baxter expanded this theory to include rough and porous surfaces in 1944 [46]. It specifies that there are air pockets among the rough grooves and that the rough surface is inherently uneven. As seen in Figure 23c, water droplets adhere to the surface rather than penetrating it. The Cassie–Baxter equation is given by where θW is the apparent contact angle in the Wenzel model, r is the factor of surface roughness, and θY is the contact angle in the Young model. According to Wenzel’s theory, when a liquid comes into contact with a rough surface, it completely fills the voids and grooves of the surface, as seen in Figure 23b; as a result, the static contact angle is decreased, and the sliding angle is increased. Wenzel’s theory states that as roughness increases, hydrophilic surfaces become more wettable, while hydrophobic surfaces become less wettable.2.4. Cassie–Baxter Model
Cassie and Baxter expanded this theory to include rough and porous surfaces in 1944 [46]. It specifies that there are air pockets among the rough grooves and that the rough surface is inherently uneven. As seen in Figure 23c, water droplets adhere to the surface rather than penetrating it. The Cassie–Baxter equation is given by where fs is the solid fraction, which means that a fraction of the solid surface is wetted by the liquid. The relationship between contact angle and surface roughness can be successfully explained by the Wenzel model as well as the Cassie–Baxter model. However, it is important to note that roughness and surface material chemistry should work together on different scales to produce hydrophobicity. Hydrophobic surface preparation, characterization, and application have recently received a lot of attention.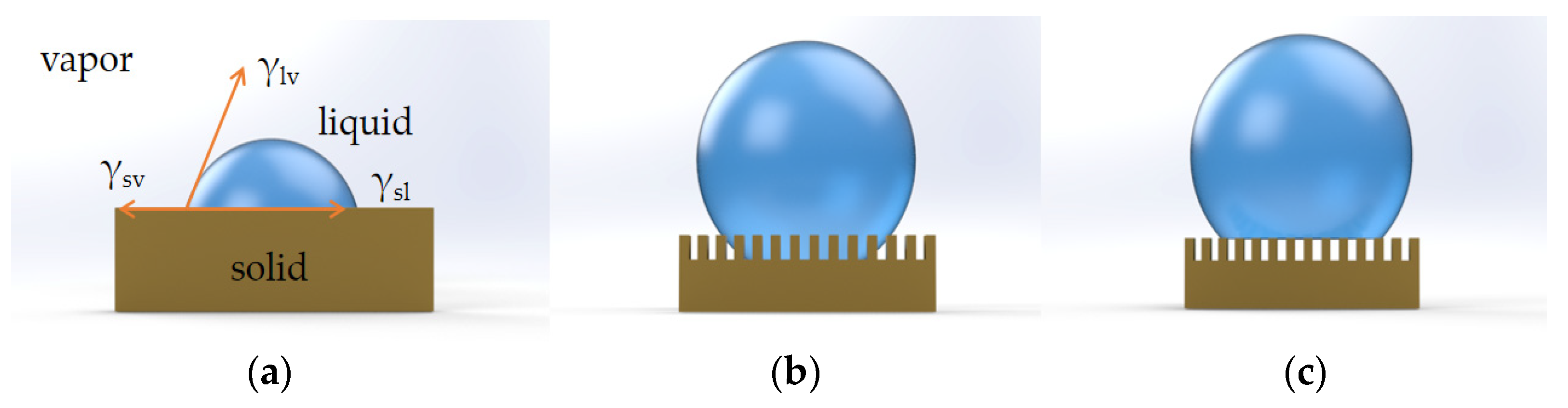
Figure 23. (a) Young model. (b) Wenzel model. (c) Cassie model.
References
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B 2015, 176, 396–428.
- Liu, K.S.; Tian, Y.; Jiang, L. Bio-inspired superoleophobic and smart materials: Design, fabrication, and application. Prog. Mater. Sci. 2013, 58, 503–564.
- Liu, M.J.; Wang, S.T.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036.
- Ma, Q.L.; Cheng, H.F.; Fane, A.G.; Wang, R.; Zhang, H. Recent Development of Advanced Materials with Special Wettability for Selective Oil/Water Separation. Small 2016, 12, 2186–2202.
- Su, B.; Tian, Y.; Jiang, L. Bioinspired Interfaces with Superwettability: From Materials to Chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748.
- Sun, Y.H.; Guo, Z.G. Recent advances of bioinspired functional materials with specific wettability: From nature and beyond nature. Nanoscale Horiz. 2019, 4, 52–76.
- Wang, S.T.; Liu, K.S.; Yao, X.; Jiang, L. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293.
- Wang, Z.X.; Elimelech, M.; Lin, S.H. Environmental Applications of Interfacial Materials with Special Wettability. Environ. Sci. Technol. 2016, 50, 2132–2150.
- Yong, J.L.; Chen, F.; Yang, Q.; Huo, J.L.; Hou, X. Superoleophobic surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217.
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M.; Van der Bruggen, B. Superhydrophilic and underwater superoleophobic membranes—Review of synthesis methods. Prog. Polym. Sci. 2019, 98, 101166.
- Bernt, D.; Ponomarenko, V.; Pisarev, A. Durability of transparent oleophobic coatings deposited by magnetron PVD. Surf. Coat. Technol. 2017, 330, 211–218.
- Xue, Z.X.; Liu, M.J.; Jiang, L. Recent developments in polymeric superoleophobic surfaces. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1209–1224.
- Liu, B.; Zhang, K.Q.; Tao, C.; Zhao, Y.H.; Li, X.H.; Zhu, K.Y.; Yuan, X.Y. Strategies for anti-icing: Low surface energy or liquid-infused? RSC Adv. 2016, 6, 70251–70260.
- Li, D.W.; Wang, H.Y.; Liu, Y.; Wei, D.S.; Zhao, Z.X. Large-scale fabrication of durable and robust super-hydrophobic spray coatings with excellent repairable and anti-corrosion performance. Chem. Eng. J. 2019, 367, 169–179.
- Li, X.W.; Shi, T.; Li, B.; Chen, X.C.; Zhang, C.W.; Guo, Z.G.; Zhang, Q.X. Subtractive manufacturing of stable hierarchical micro-nano structures on AA5052 sheet with enhanced water repellence and durable corrosion resistance. Mater. Des. 2019, 183, 108152.
- Badaraev, A.D.; Sidelev, D.V.; Kozelskaya, A.I.; Bolbasov, E.N.; Tran, T.H.; Nashchekin, A.V.; Malashicheva, A.B.; Rutkowski, S.; Tverdokhlebov, S.I. Surface Modification of Electrospun Bioresorbable and Biostable Scaffolds by Pulsed DC Magnetron Sputtering of Titanium for Gingival Tissue Regeneration. Polymers 2022, 14, 19.
- Belgroune, A.; Aissani, L.; Alhussein, A.; Zaabat, M.; Kiwi, J.; Rtimi, S. Bacterial inactivation on sputtered TiOMoN and TiOMoN-Ag thin films under solar simulated light. Chem. Eng. J. 2023, 460, 13.
- Patel, N.P.; Chauhan, K.V. Effect of sputtering power and substrate temperature on structural, optical, wettability and anti-icing characteristics of aluminium doped zinc oxide. Mater. Res. Express 2022, 9, 9.
- Patel, N.P.; Chauhan, K. Impact of deposition time and working pressure on delay of ice formation on aluminum doped zinc oxide thin films. Thin Solid Films 2023, 769, 7.
- Hou, H.F.; Chen, Y.Q. Preparation of super-hydrophobic silica films with visible light transmission using phase separation. J. Sol-Gel Sci. Technol. 2007, 43, 53–57.
- Kim, Y.; Lee, J.H.; Kim, K.J.; Lee, Y. Surface characterization of hydrophobic thin films deposited by inductively coupled and pulsed plasmas. J. Vac. Sci. Technol. A 2009, 27, 900–906.
- Dimitrakellis, P.; Gogolides, E. Hydrophobic and superhydrophobic surfaces fabricated using atmospheric pressure cold plasma technology: A review. Adv. Colloid Interface Sci. 2018, 254, 1–21.
- Kim, H.M.; Sohn, S.; Ahn, J.S. Transparent and super-hydrophobic properties of PTFE films coated on glass substrate using RF-magnetron sputtering and Cat-CVD methods. Surf. Coat. Technol. 2013, 228, S389–S392.
- Martin-Palma, R.J.; Pantano, C.G. Ultra-thin hafnium oxide coatings grown by atomic layer deposition: Hydrophobicity/hydrophilicity over time. Mater. Res. Express 2019, 6, 086457.
- Ozkucur, N.; Wetzel, C.; Hollstein, F.; Richter, E.; Funk, R.H.W.; Monsees, T.K. Physical vapor deposition of zirconium or titanium thin films on flexible polyurethane highly support adhesion and physiology of human endothelial cells. J. Biomed. Mater. Res. Part A 2009, 89A, 57–67.
- Wan, Y.; Chao, W.L.; Liu, Y.F.; Zhang, J.Y. Tribological performance of fluoroalkylsilane modification of sol-gel TiO2 coating. J. Sol-Gel Sci. Technol. 2011, 57, 193–197.
- Poddighe, M.; Innocenzi, P. Hydrophobic Thin Films from Sol-Gel Processing: A Critical Review. Materials 2021, 14, 6799.
- Moganapriya, C.; Rajasekar, R.; Mohanraj, T.; Gobinath, V.K.; Kumar, P.S.; Poongodi, C. Dry Machining Performance Studies on TiAlSiN Coated Inserts in Turning of AISI 420 Martensitic Stainless Steel and Multi-Criteria Decision Making Using Taguchi-DEAR Approach. Silicon 2022, 14, 4183–4196.
- Moganapriya, C.; Rajasekar, R.; Santhosh, R.; Saran, S.; Santhosh, S.; Gobinath, V.K.; Kumar, P.S. Sustainable Hard Machining of AISI 304 Stainless Steel Through TiAlN, AlTiN, and TiAlSiN Coating and Multi-Criteria Decision Making Using Grey Fuzzy Coupled Taguchi Method. J. Mater. Eng. Perform. 2022, 31, 7302–7314.
- Moganapriya, C.; Rajasekar, R.; Ponappa, K.; Venkatesh, R.; Karthick, R. Influence of cutting fluid flow rate and cutting parameters on the surface roughness and flank wear of tialn coated tool in turning aisi 1015 steel using taguchi method. Arch. Metall. Mater. 2017, 62, 1827–1832.
- Moganapriya, C.; Rajasekar, R.; Ponappa, K.; Venkatesh, R.; Jerome, S. Influence of Coating Material and Cutting Parameters on Surface Roughness and Material Removal Rate in Turning Process Using Taguchi Method. In Proceedings of the 1st International Conference on Emerging Trends in Materials and Manufacturing Engineering (IMME), Tiruchirappalli, India, 10–12 March 2017; pp. 8532–8538.
- Betz, U.; Olsson, M.K.; Marthy, J.; Escola, M.F.; Atamny, F. Thin films engineering of indium tin oxide: Large area flat panel displays application. Surf. Coat. Technol. 2006, 200, 5751–5759.
- Minami, T. Transparent conducting oxide semiconductors for transparent electrodes. Semicond. Sci. Technol. 2005, 20, S35–S44.
- Takeda, S.; Suzuki, S.; Odaka, H.; Hosono, H. Photocatalytic TiO2 thin film deposited onto glass by DC magnetron sputtering. Thin Solid Films 2001, 392, 338–344.
- Ellmer, K. Magnetron sputtering of transparent conductive zinc oxide: Relation between the sputtering parameters and the electronic properties. J. Phys. D Appl. Phys. 2000, 33, R17–R32.
- Chung, S.I.; Kim, P.K.; Ha, T.G. Fabricating transparent nanomesh-structured hydrophobic films by nanoimprinting UV-curable fluorinated polyurethane acrylates. J. Micromech. Microeng. 2020, 30, 8.
- Saji, V.S. Superhydrophobic surfaces and coatings by electrochemical anodic oxidation and plasma electrolytic oxidation. Adv. Colloid Interface Sci. 2020, 283, 27.
- Feng, L.; Li, S.H.; Li, Y.S.; Li, H.J.; Zhang, L.J.; Zhai, J.; Song, Y.L.; Liu, B.Q.; Jiang, L.; Zhu, D.B. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860.
- Feng, X.J.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078.
- Gao, X.F.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36.
- Feng, L.; Zhang, Y.A.; Xi, J.M.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119.
- Yang, S.; Ju, J.; Qiu, Y.C.; He, Y.X.; Wang, X.L.; Dou, S.X.; Liu, K.S.; Jiang, L. Peanut Leaf Inspired Multifunctional Surfaces. Small 2014, 10, 294–299.
- Rajasekar, R.; Kim, N.H.; Jung, D.; Kuila, T.; Lim, J.K.; Park, M.J.; Lee, J.H. Electrostatically assembled layer-by-layer composites containing graphene oxide for enhanced hydrogen gas barrier application. Compos. Sci. Technol. 2013, 89, 167–174.
- Young, T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87.
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994.
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551.
More
