1. Introduction
Tumor Suppressor Candidate 2 (
TUSC2/
FUS1) was first discovered as a candidate tumor suppressor gene (TSG) within the 630-kb homozygous deletion on chromosomal region 3p21.3 in lung cancer
[1]. This chromosomal region was first identified as one of the four allele regions lost on chromosomal region 3p in both small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) cases
[2][3][4][2,3,4]. The 3p21.3 region was studied specifically for its frequent deletion in preneoplastic lung cancer lesions in current and former smokers, suggesting that the 3p21.3 region contains a cluster of TSGs whose loss contributes to lung cancer initiation
[2][3][5][6][2,3,5,6]. Further analysis narrowed the region of interest to a critical gene region of 370 kb, and even further to a 120 kb region most likely containing critical TSGs deleted in both breast and lung cancer
[7][8][7,8]. Through generation of multiple cosmid cloning vectors of the 630-kb clone region followed by Sanger sequencing, Lerman et al. discovered 25 resident genes and multiple candidate genes in the 3p21.3 region, with 10 genes located in the 370 kb segment of the critical gene region, and 9 within the 120 kb segment of the same region. Of these 25 resident genes, they determined that
TUSC2/FUS1 is one of the potential TSGs located within the 120 kb segment of the 370 kb critical gene region.
TUSC2/FUS1 (herein referred to as
TUSC2) was named for its positioning as the “fusion” junction between cosmid LUCA12 and LUCA13
[1].
TUSC2 is a 3.3 kb gene that contains three exons, which are transcribed into a 1.8 kb mRNA transcript that is abundantly expressed in all human tissues
[1]. Translation of
TUSC2 mRNA produces a 110 amino acid protein consisting of multiple functional domains including a myristoylation motif (1–9 aa), myristoyl binding motif (45–110 aa), and an EF-hand calcium-binding motif (54–66 aa)
[1][9][10][1,9,10]. Two post-translational modifications (PTMs) are frequently found on TUSC2, including N-terminal myristoylation and poly-ubiquitination of TUSC2 lysine residues K71, K84, and K93
[9][11][9,11]. TUSC2 N-terminal myristoylation covalently adds a 14-carbon myristoyl group to the N-terminal glycine residue (G2) during protein translation
[9][12][9,12]. N-terminal myristoylated proteins have critical biological functions, including calcium and ion-channel regulation, controlling protein localization and altering cellular structure and the regulation of protein–protein and protein–substrate interactions
[13][14][15][13,14,15]. Uno et al. found N-terminally myristoylated TUSC2 to induce apoptosis, suppress cell growth, and suppress tumor xenografts and lung metastasis in vivo. Conversely, unmyristoylated TUSC2 had significantly reduced tumor suppressive function, resulting in reduced TUSC2 mediated apoptosis and cell cycle inhibition in lung cancer. Moreover, they found unmyristoylated TUSC2 to be more susceptible to proteasomal degradation in lung cancer. Furthermore, they generated a myristoylation deficient TUSC2 mutant, Myr-mut-FUS1, which resulted in a shorter duration of transient protein expression and had a protein half-life of 6 h compared to the 12 h of wt-FUS1 (wild type TUSC2). Additionally, Myr-mut-FUS1 protein half-life was stabilized and comparable to wt-FUS1 upon inhibition of the proteasome, demonstrating that FUS1 (TUSC2) protein stability is regulated through the ubiquitin proteasomal degradation system
[9]. Proteins are most commonly marked for proteasomal degradation via protein polyubiquitination
[16]. TUSC2 is poly-ubiquitinated on lysine residues K71, K84, and K93 in glioblastoma (GBM)
[11].
2. TUSC2 Normal Cellular Functions
2.1. TUSC2 and Calcium Regulation
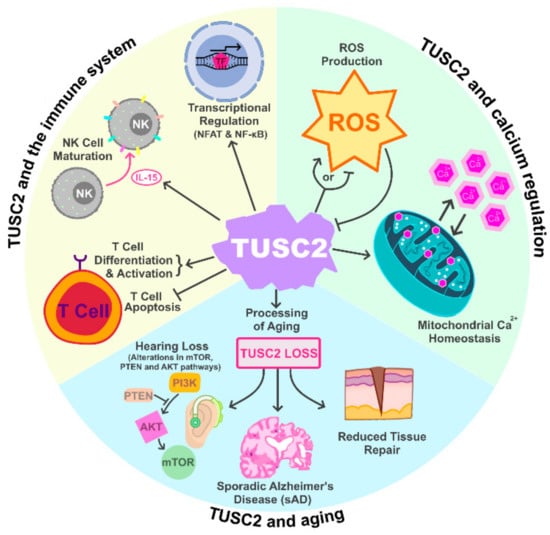
TUSC2 is a major regulator of mitochondrial calcium handling and intracellular calcium homeostasis (
Figure 1)
[10]. The TUSC2 amino acid sequence contains a highly homologous region to an EF-hand calcium binding domain, spanning from amino acids 54 to 65
[10][17][10,21]. The EF-hand motif is the most common calcium-binding motif found in calcium binding proteins and is found in more than 66 subfamilies of calcium-binding proteins, including the proteins calmodulin and troponin
[13][18][19][20][13,22,23,24]. Calmodulin and troponin are EF-hand calcium-binding proteins that mediate calcium (Ca
2+) movement for intracellular calcium dependent signaling pathways and cardiac and skeletal muscle contraction respectively
[17][18][19][21,22,23]. Calcium ions are involved in a multitude of cellular processes, including altering protein conformation, muscle contraction, cell proliferation, cell development, and cell death
[21][22][23][25,26,27]. Ca
2+ is also important for overall mitochondrial function and regulation
[24][25][28,29]. Ca
2+ influx can lead to mitochondrial membrane depolarization, or it can be used as a buffer for overall cellular Ca
2+ homeostasis
[24][28]. Recoverin is one such calcium-binding protein that contains multiple EF-hand calcium binding motifs, an N-terminal myristoylation site, and is classified as a calcium/myristoyl switch protein
[13][14][26][27][28][13,14,30,31,32]. Upon binding of Ca
2+ to two of the four EF-hand motifs on recoverin, N-terminal myristoylated recoverin “switches” and releases the hydrophobic myristoyl group from its myristoyl binding pocket, which anchors recoverin into cellular membranes
[13][29][13,33]. Sequence and protein homology analyses determined that TUSC2 shares the highest homology with the recoverin protein, with TUSC2 sharing 53% homology to the myristoyl binding domain of recoverin
[10]. Due to its homology to recoverin, as well as the identification of its N-terminal myristoylation and EF-hand motif, TUSC2 can be classified as a calcium/myristoyl switch protein
[9][10][13][14][26][9,10,13,14,30]. Additionally, the N-terminal myristoylation is important for TUSC2 subcellular membrane localization, specifically in the mitochondria
[9][10][9,10].
Figure 1. TUSC2 Normal Cellular Functions. TUSC2 has been shown to play important roles in regulating the immune system, cellular calcium regulation as well as the process of aging.
TUSC2 is also important for regulating mitochondrial calcium homeostasis
[10][30][10,34]. Uzhachenko et al. illustrated that TUSC2 knockout (TUSC2-KO) elevates mitochondrial calcium levels and disrupts the ability of mitochondria to properly accumulate calcium following treatment with ionomycin, an ionophore that binds free floating calcium, in epithelial and immune cells. This dysregulation of mitochondrial calcium accumulation results from excessive Ca
2+ efflux from the mitochondrial Na
+/Ca
2+ exchanger following TUSC2-KO
[10][31][10,35]. Furthermore, TUSC2 loss and altered mitochondrial calcium regulation result in increased mitochondrial fission and fragmentation, which signifies mitochondrial stress
[10][32][10,36].
Mitochondrial calcium homeostasis is important in regulating overall mitochondrial function and for regulating the formation of reactive oxygen species (ROS)
[33][37]. Interestingly, TUSC2 expression has been shown to be downregulated by ROS, and ROS levels has been found to be regulated by TUSC2, suggesting a potential feedback or regulatory loop between TUSC2 and mitochondrial ROS production
[30][34][35][17,34,38]. Whether TUSC2 promotes or inhibits ROS production is dependent on cell type. For example, in innate immune TUSC2-KO cell populations, TUSC2 loss results in a significant decrease in ROS production, whereas ROS production was significantly elevated in TUSC2-KO B and T cells, suggesting that TUSC2 regulates mitochondrial ROS homeostasis
[30][34]. Additionally, TUSC2 loss has been found to increases ROS production in multiple cancer lines
[30][34]. Elevated ROS levels influence multiple stages of cancer progression, including tumorigenesis, metastasis, and cell survival
[36][37][38][39,40,41]. However, high levels of ROS may promote cancer cell apoptosis
[39][42].
2.2. TUSC2 and the Immune System
A previous report by Ivanova et al. demonstrated that mice with conventional TUSC2-KO exhibited altered overall immune responses, suggesting that TUSC2 may regulate processes within the immune system (
Figure 1)
[40][43]. Ivanova and colleagues also found that mice with TUSC2 heterozygous and TUSC2 homozygous KO displayed greater mortality between the ages of two and 14 months compared to their wild-type littermates, with 11% of either heterozygous or homozygous TUSC2-KO cohorts dying or reaching humane endpoints. Deceased TUSC2-KO mice frequently showed signs of systemic infection, fibrinoid necrotizing arteritis in multiple organs, severe nephropathy, anemia, and glomerulonephritis, which are pathologies associated with autoimmune disorders such as systemic lupus erythematosus
[41][42][44,45]. Additionally, deceased TUSC2-KO mice frequently exhibited spontaneous tumors, including hemangioma, hemangiosarcoma, lymphoma, and other spontaneous malignancies, indicating that TUSC2 loss may play a role in tumorigenesis. At two years of age, 23% of TUSC2 heterozygous KO and 20% of TUSC2 homozygous KO mice had multi-organ arteritis compared to WT mice. Additionally, TUSC2-KO mice also had high levels of circulating autoreacting nuclear antibodies, further implicating TUSC2 as an important regulator of the immune system and TUSC2 loss as a potential driver of autoimmune disorder development
[40][43].
In addition to regulating autoimmune disorder development, TUSC2 has been implicated in the immune response and inflammation in an asbestos-mesothelioma TUSC2-KO mouse study
[30][34]. Uzhachenko et al. demonstrated that TUSC2 loss is associated with an increased inflammatory response following injection of asbestos, a well-documented irritant that promotes inflammation, into TUSC2-KO mice. Conventional TUSC2-KO mice displayed reduced spleen and liver mass as well as altered protein accumulation in the peritoneal fluid following asbestos injection, demonstrating an overall altered immune response compared to TUSC2-WT mice. Furthermore, histopathological analyses revealed that TUSC2-KO mice exposed to asbestos had inflamed mesenteric fat with prominent infiltration of fibroblasts and Langhans-type giant cells, as well as increased positive Ki-67 staining in the mesothelial cells, demonstrating signs of chronic inflammation. TUSC2-KO mice also demonstrated a significant increase in pro-inflammatory cytokines
IFNγ and
IL-1α, with a significant decrease in regulatory cytokines such as
IL-10. Reduced T cell activation, as well as altered ROS levels and mitochondrial membrane potential in innate and adaptive immune cells, are also reported as a result of TUSC2-KO in mice, further demonstrating the importance of TUSC2 in immune cell function
[30][34].
TUSC2 is highly expressed in human T and B cells, with increased expression in activated T cells
[40][43]. TUSC2 regulates T cell activation and differentiation by promoting expression of the vital T cell surface markers CD4, PD-1, and PD-L1
[10][43][10,46]. Conversely, TUSC2 loss significantly decreases the surface expression of T cell surface markers, thereby reducing T cell activation and differentiation
[10]. Concordantly, TUSC2 restoration in lung cancer cell lines decreased PD-L1 expression, promoting an anti-tumor immune response and enhancing the efficacy of anti-PD-1 and anti-PD-L1 therapies
[44][45][46][47][47,48,49,50]. Alternatively, TUSC2 loss leads to impaired mitochondrial calcium handling and results in the downregulation of many genes required for T cell activation via the CD3/CD28 receptor pathway, including
TNF-α, IRF4, and
IL-2 [10]. Furthermore, TUSC2 inhibits T cell proliferation while having no effect on T cell apoptosis, and is hypothesized to inhibit Th1 differentiation following CD3/CD28 stimulation, which strongly suggests that TUSC2 regulates overall T cell function
[10].
TUSC2 also promotes natural killer (NK) cell maturation
[40][43]. In the conventional TUSC2-KO mouse model generated by Ivanova et al., TUSC2-KO mice had a 45% decrease in total NK cell numbers and a 57% decrease in their percentage of mature NK cells as compared to WT mice. Moreover, TUSC2-KO mice had significantly lower levels of the cytokine IL-15, a critical cytokine for NK cell maturation
[40][43]. Treatment with IL-15 restored the mature NK cell population in TUSC2-KO mice, indicating that IL-15 may be an important factor downstream of TUSC2 for promoting NK cell maturation
[34][40][17,43]. IL-15 expression was later found to be significantly increased following TUSC2 overexpression, supporting the role of TUSC2 in regulating IL-15 expression and the importance of TUSC2 in NK cell maturation
[34][17].
In addition to IL-15, TUSC2 regulates the expression of multiple immune-related genes
[10][30][34][43][10,17,34,46]. TUSC2 overexpression in mesothelioma cells altered expression of 46 immune-related genes, whereas the expression of
IFNγ, IFNγR, IL-1α, IL-1β, CCL5, and
IL-10 was significantly increased in immune cells derived from a conventional TUSC2-KO mouse model
[30][34][17,34]. These findings indicate that TUSC2 mediated immune regulation may arise from altered expression of multiple downstream immune related genes
[30][34][17,34]. Additionally, Uzhachenko et al. discovered that TUSC2 regulates the calcium-dependent transcription factor NFAT (nuclear factor of activated T-cells) and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), which are transcription factors that regulate inflammation
[10]. Genes suppressed by TUSC2 in T-cells have promoters that are enriched in binding sites for both NFAT and NF-κB, as well as binding sites for NFAT-cooperating transcription factors MAF, IRF, and OCT1
[10]. Furthermore, many of the genes upregulated in TUSC2-KO T cells, such as
resistin (which promotes NF-κB activation) and
S100a8/S100a9, which are key genes in promoting the inflammation associated with rheumatoid arthritis and inflammatory bowel disease, are pro-inflammatory
[10][48][10,51]. Many downstream genes regulated by TUSC2 and NFAT/NF-κB are involved in extracellular matrix remodeling or overall cellular calcium handling, suggesting that the TUSC2/NFAT/NF-κB pathway may regulate important calcium controlled immune functions
[10]. Interestingly, TUSC2 also regulates the NF-κB pathway in osteoclasts through increasing the activation of receptor activator of nuclear factor κB ligand (RANKL), promoting bone reabsorption
[43][46]. Overall, TUSC2 functions as an important factor involved in overall immune system regulation through multiple downstream immune related genes, specifically in T, B, and NK cells, controlling the innate immune response and the development of autoimmune disorders.
2.3. TUSC2 and Aging
Studies have also found TUSC2 to be involved in the processes of aging (
Figure 1). In a conventional TUSC2-KO mouse model, Coronas-Somano et al. found that TUSC2 loss induced sporadic Alzheimer’s Disease (sAD) in mice. TUSC2-KO mice presented with multiple cellular alterations associated with cognitive and olfactory sAD symptoms, such as disrupted mitochondrial homeostasis with increased oxidative stress and altered calcium signaling, as well as increased autophagy within the brain. Along with signaling pathway alterations, TUSC2-KO mice demonstrated deficits in olfactory and spatial memory and showed longer sleep times during the diurnal cycle
[49][52]. In a follow up study, Uzhachenko et al. found that TUSC2-KO mice also presented with multiple symptoms of premature aging such as lordokyphosis, which is an arched or curved spine; reduced stress tolerance; lack of vigor; and premature death. TUSC2-KO mice also presented with low sperm count, chronic inflammation, as well as a reduced ability to repair tissue damage. The mitochondria of TUSC2-KO mice showed altered dynamics in calcium accumulation and had an overall lower ability to produce energy quickly
[50][53]. Additionally, Tan and colleagues also found TUSC2 loss to be associated with age-related hearing loss resulting from changes in cellular metabolism. The hearing loss is attributed to alterations in the antioxidant and nutrient and energy sensing pathways of mTOR, PTEN, and AKT within the cochleae of the TUSC2-KO mice. Interestingly, treatment of TUSC2-KO mice with an antioxidant treatment, N-acetyl cysteine, resulted in molecular changes that restored hearing responses, mitochondrial structure, and delayed the onset of hearing loss in TUSC2-KO mice
[51][54]. A similar TUSC2-KO mouse study demonstrated a role for TUSC2 in radioprotection
[52][55]. Yazlovitskaya et al. showed that TUSC2-KO mice had higher mortality rates following radiation as compared to the TUSC2-KO mice. Moreover, GI crypt epithelial cells of the TUSC2-KO mice were more apoptotic and exhibited dysregulated cellular pathways involved in immune response, oxidative stress response, apoptosis, cell cycle, and DNA repair
[52][55]. Thus, TUSC2 loss is associated with multiple phenotypes of premature aging, including sAD, reduced tissue repair, hearing loss, altered antioxidant and nutrient and energy signaling pathways, and radiosensitivity in TUSC2-KO mice.
3. TUSC2 in Cancer
3.1 TUSC2 loss in Cancers
Since its discovery, TUSC2 loss has been found in a multitude of cancers ranging from lung, breast, ovarian, thyroid, melanoma, glioma and other cancer types [9][11][53][34][54][55][9,11,17-20]. TUSC2 loss in cancer is mediated by somatic deletion, transcriptional downregulation, post-transcriptional downregulation via miRNAs, and post-translationally through the proteasomal degradation system [1][9][11][56][57][1,9,11,64,65]. Across multiple cancer types, restoration of TUSC2 expression promotes tumor suppression through reduced cell proliferation, increased apoptosis and reduced tumor growth in vivo [11][55][58][59][11,20,72,75]. Although TUSC2 is tumor suppressive across multiple cancer types, the exact mechanisms of TUSC2 mediated tumor suppression are still poorly understood. However, TUSC2 has been shown to indirectly regulate expression of multiple downstream EGFR target genes, such as FGFR2, mTOR, AKT, and c-Abl in lung cancer [58][59][60][61][72,75-77]. Additionally, TUSC2 overexpression was found to reduce MDM2 expression, promoting protein stability of the tumor suppressor p53 [62][79]. Furthermore, TUSC2 was found to indirectly regulate expression of anti-apoptotic protein Bcl-xL in GBM [11]. Although the mechanisms of TUSC2 mediated tumor suppression are poorly understood, TUSC2 is a well established tumor suppressor across multiple cancer types that warrants further investigation into its mechanisms of action.
3.2 TUSC2 Cancer Therapy
Current anti-cancer drug therapies are primarily aimed at inhibiting target oncogenes to impede cancer growth and progression. However, therapies that aim to restore expression or replace function of lost tumor suppressors are under-developed. Designing treatments to either increase tumor suppressor protein expression, or to replace the tumor suppressor function is especially challenging with conventional drug discoveries and current therapeutics in cancer. Nevertheless, restoration of tumor suppressor expression through targeted nanoparticle delivery systems has shown promise as a potential therapeutic strategy, as seen with TUSC2 and lung cancer
[63][106]. In 2012, a Phase I clinical trial (NCT00059605) was conducted on 31 recurrent or metastatic lung cancer patients previously treated with platinum based chemotherapy, in which they were treated with
N-[1-(2,3-dioleoyloxy)propyl]-
N,
N,
N-trimethylammonium chloride (DOTAP):cholesterol nanoparticles containing a TUSC2 expression plasmid
[63][106]. Patients were treated intravenously (IV) every three weeks with one of six doses (ranging from 0.01 to 0.09 mg/kg) of the TUSC2 carrying nanoparticle, showing a maximum tolerated dose of 0.06 mg/kg. The trial resulted in five patients achieving stable disease, as well as a large portion of patients successfully having increased TUSC2 mRNA and protein expression in post-treatment biopsy samples. Additionally, multiple apoptotic proteins involved in the intrinsic apoptosis pathway were significantly upregulated in patients post-treatment. The results from this trial demonstrated that treatments with nanoparticles carrying a TUSC2 expression plasmid are able to be safely administered through IV treatment and result in increased expression of the tumor suppressor TUSC2, as well as upregulation of pro-apoptotic genes in recurrent and metastatic lung cancer patients, with the potential of stabilizing disease progression
[63][106]. In fact, a clinical trial is currently in progress using the TUSC2 expression plasmid containing DOTAP:cholesterol nanoparticles (REQORSA) in combination with EGFR inhibitor Osimertinib (NCT04486833) in advanced lung cancer patients who experienced disease progression on Osimertinib alone
[64][107]. The previous and current clinical trial are providing the necessary groundwork to enhance targeted cancer therapy utilizing tumor suppressor genes.